Continuing Education Activity
Lacrimal gland masses can arise from many etiologies, including neoplastic and non-neoplastic causes. Of the neoplastic causes, the malignant tumors generally have a poor prognosis and thus are treated aggressively. This usually involves a combination of extensive surgical resection (often orbital exenteration), radiation, and chemotherapy. The most notable exception is orbital lymphoma, which is not treated surgically. Recent developments in treatment strategies involving intra-arterial chemotherapy prior to surgical resection of adenoid cystic carcinoma, the most common malignant epithelial lacrimal gland tumor, may offer a new direction for improving patient outcomes. This activity reviews the evaluation and treatment of malignant lacrimal gland tumors and explains the role of the interprofessional team in managing patients with these conditions.
Objectives:
- Describe the presentation and appropriate clinical evaluation for a patient with a suspected malignant lacrimal fossa mass.
- Review the malignant causes of lacrimal gland tumors.
- Identify factors of the history, physical, or imaging modalities that guide management and treatment.
- Summarize the treatment strategies by the interprofessional team for the various malignant etiologies.
Introduction
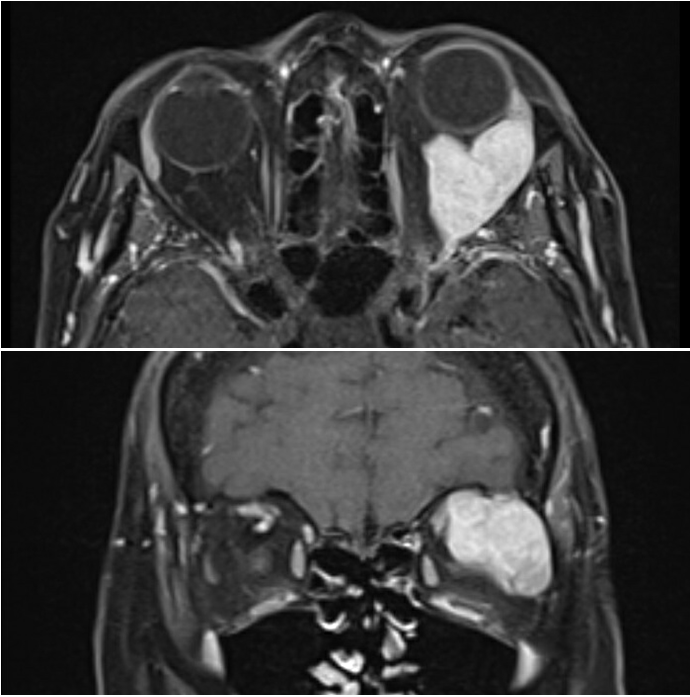
Tumors of the lacrimal gland fossa account for about 10% of all biopsied orbital masses.[1] There is a wide variety of etiologies for these masses, including infectious, inflammatory, and neoplastic.[1][2] Furthermore, neoplastic lesions can either be benign or malignant. This activity will focus on malignant lacrimal gland tumors (broadly called lacrimal gland carcinomas). Since the lacrimal gland fossa is in the anterior superolateral orbit, space-occupying lesions of this area of the orbit typically cause inferior and medial globe displacement.
Proptosis is not always seen but can be present if the posterior growth of a mass pushes the eye forward. Most malignant lesions grow quicker than benign lesions, averaging less than six months from onset to diagnosis compared to 1 to 2 years for benign lesions.[3] Malignant lacrimal gland lesions generally carry a poor prognosis; thus quick, and early identification is critical to improving a patient's morbidity and mortality.
Etiology
Tumors of the lacrimal gland are similar to tumors of the salivary glands due to their shared embryologic origins. The type of proliferating cell can organize the types of tumors, and the most common distinction is epithelial versus non-epithelial.[4]
Epithelial
Nearly 50% of epithelial tumors of the lacrimal gland are malignant, though this may depend on referral patterns, geographic location, and patient population.[1][5]
Adenoid cystic carcinoma (ACC) is the most common malignant epithelial tumor, comprising 66% of malignant epithelial tumors. It commonly presents with globe displacement, proptosis, and ptosis; pain and frontotemporal numbness are more specific symptoms as adenoid cystic carcinoma is classically known for invading orbital nerves.[6]
Carcinoma ex pleomorphic adenoma (CEPA, also known as pleomorphic adenocarcinoma or malignant mixed tumor) is the second most common type of epithelial malignancy and, as the name suggests, transforms from a benign pleomorphic adenoma tumor, either from a previously biopsied or resected tumor (if taken piecemeal or if the capsule was ruptured during removal), or de novo from a primary pleomorphic adenoma without prior surgery.[2][7] This transformation can occur years or decades after the primary pleomorphic adenoma is treated. The malignant component can be any epithelial cell, but most commonly is adenocarcinoma not-otherwise-specified or mucoepidermoid carcinoma.
Mucoepidermoid carcinoma (MEC) and adenocarcinoma not-otherwise-specified (NOS) are the next most common types. Mucoepidermoid carcinoma is a common salivary gland tumor but a rare lacrimal gland tumor. Adenocarcinoma NOS is a primary adenocarcinoma of the lacrimal gland that does not have features of the other listed types. Other epithelial malignancies include ductal carcinoma (similar to mammary ductal carcinoma), acinic cell carcinoma, myoepithelial carcinoma, epithelial-myoepithelial carcinoma, cystadenocarcinoma, carcinosarcoma, polymorphous low-grade adenocarcinoma, sebaceous cell carcinoma, squamous cell carcinoma, and basal cell carcinoma.
Lymphoid
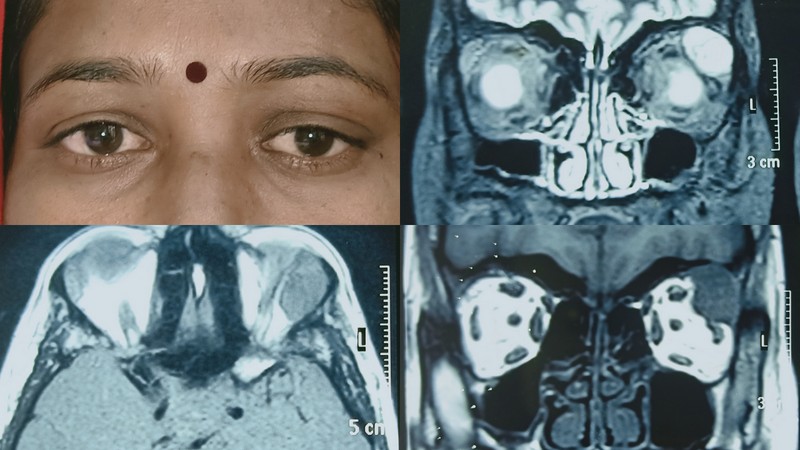
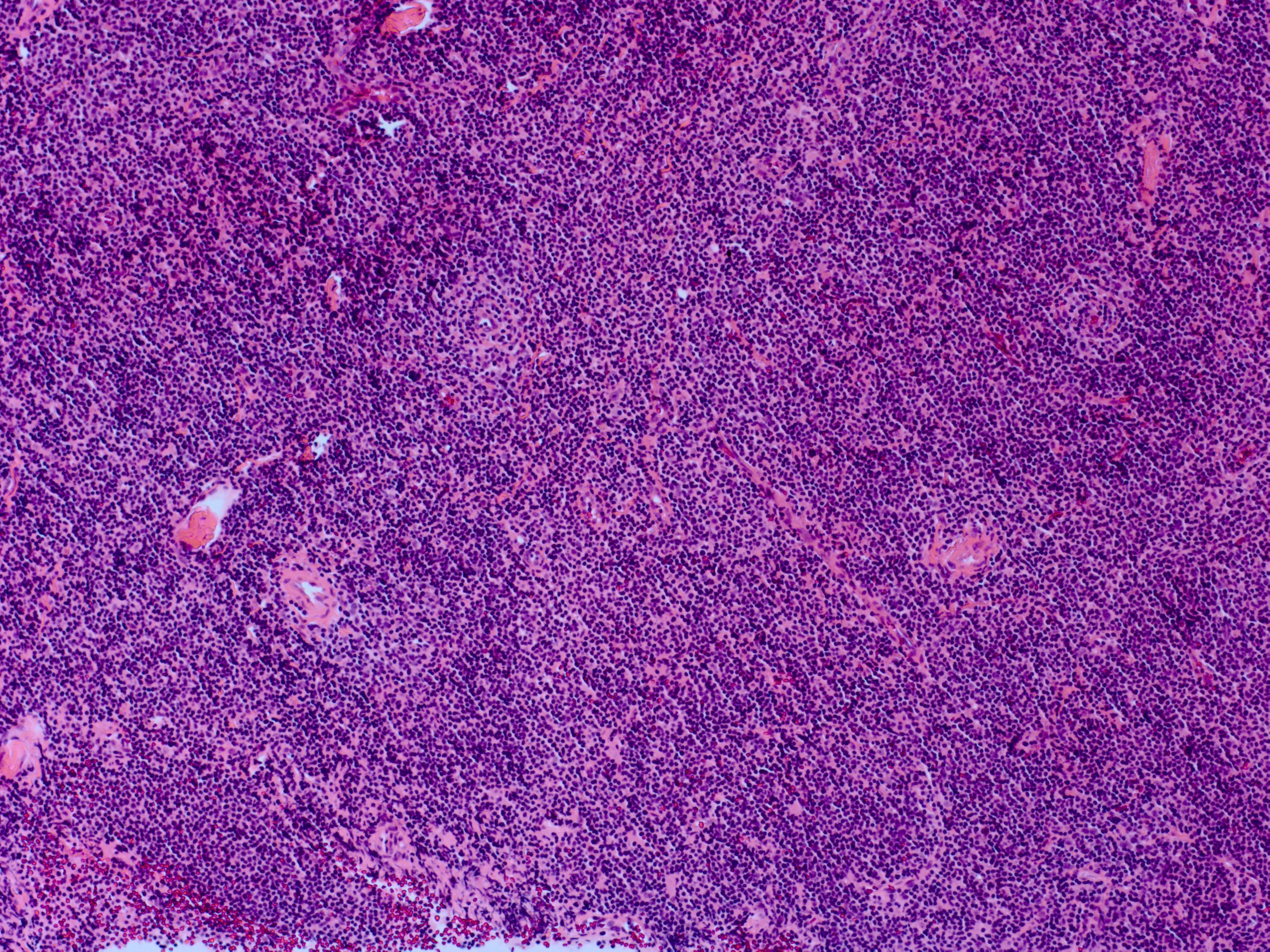
The overwhelming majority of lacrimal gland lymphomas are non-Hodgkin's B cell lymphomas. The most common subtype is extranodal marginal zone lymphoma (EMZL, MALT lymphoma) which makes up to 40% of lacrimal gland lymphomas. It also may be associated with inflammatory diseases such as reactive (benign) lymphoid hyperplasia, IgG4-related disease, Sjogren syndrome, and gastric H. pylori infection.[8][9]
Other subtypes include follicular lymphoma, diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma. Lymphomas present with painless growth and usually minimal signs of inflammation. Orbital lesions often precede systemic lymphoma presentation, but the disease may be isolated to just the orbit without every developing systemic disease.
Mesenchymal
Malignant mesenchymal tumors of the lacrimal gland are very rare, with vascular lesions most common.[4]
Metastasis
Metastases to the lacrimal gland from systemic cancers are exceedingly rare since there are no lymph nodes in the lacrimal gland. These cases are described in the literature in isolated case reports, and the most common primary site is the breast.[8][10]
Epidemiology
In general, lacrimal gland carcinomas do not show a gender preference, though some exceptions exist. Adenocarcinoma NOS and ductal carcinoma have a slight predilection for males, whereas MEC and lymphoma have a slight female predilection.[11][12]
All types of malignant lesions can appear at any age, but most are typically found in the older adult age group (6th decade or later).[5] The notable exception to this is adenoid cystic carcinoma, which has an average age of diagnosis of 40 years. There is no racial or geographic preference for tumors, though this may be because they are rare overall.
Pathophysiology
Malignant tumors arise from genetic changes involving oncogenes and tumor suppressor genes. Some of these changes have been characterized by specific lacrimal gland neoplasms. For adenoid cystic carcinoma, abnormalities in MYB or MYB-NFIB protein expression have been identified and may serve as a critical event in the oncogenic process.
Carcinoma ex pleomorphic adenoma tumors likely have the same underlying genetic abnormalities as pleomorphic adenomas, such as PLAG1, and the genetic abnormalities were seen in the corresponding tumor's malignant component. Mucoepidermoid carcinoma of the salivary gland has been shown to harbor a translocation between chromosomes 11 and 19, resulting in a fusion of oncogenes CRTC1 and MAML2, and it has been found that lacrimal gland MEC tumors are similar.[13]
Ductal carcinomas frequently exhibit HER2 and androgen receptor positivity and are always ER and PR negative. For lymphoma, EMZL exhibits a translocation between chromosomes 14 and 18 or between chromosomes 3 and 14, follicular lymphoma has translocations between chromosomes 14 and 18, and mantle cell lymphoma has translocations between chromosomes 11 and 14.[8]
Histopathology
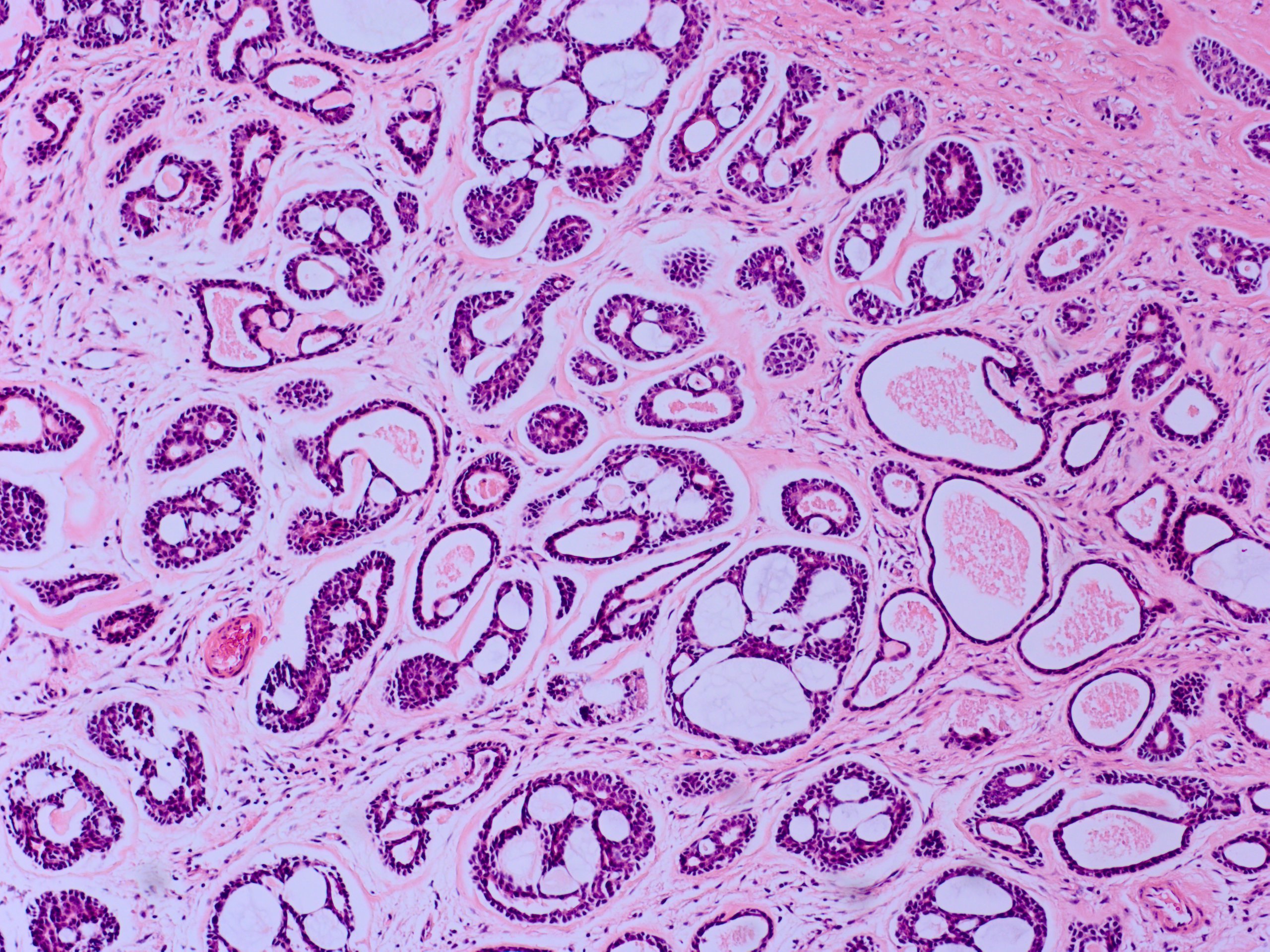
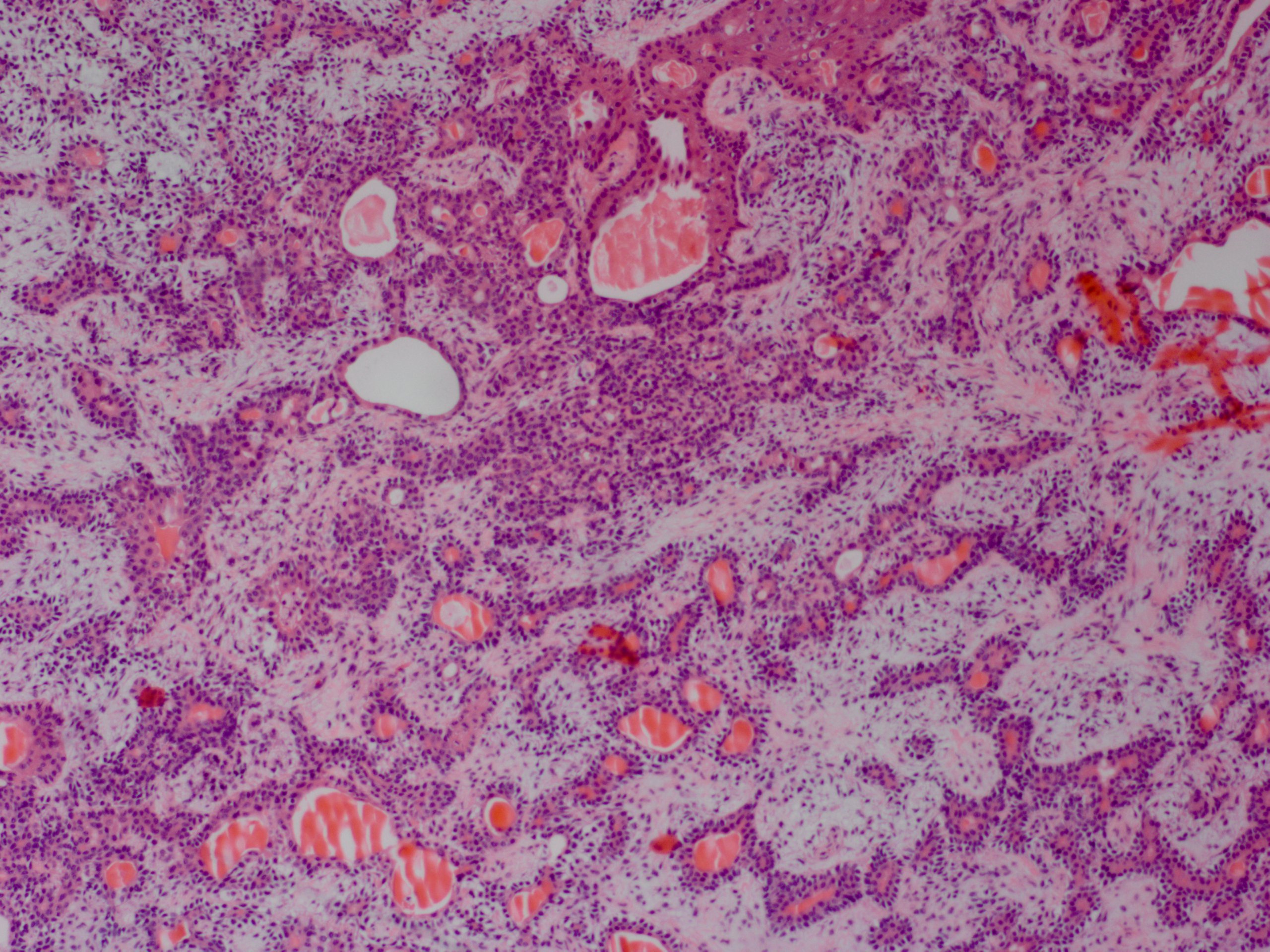
Adenoid cystic carcinoma has multiple histological forms. The most common is the cribriform (Swiss cheese) pattern with nests of myoepithelial and ductal cells with cylindrical cystic structures filled with hyaline basal lamina or basophilic mucoid (glycosaminoglycan) material.[3] The other forms are tubular and solid, and usually, there is a mix of the different forms within one tumor. The neoplasm is usually unencapsulated and shows infiltration into the surrounding tissue. Perineural growth is characteristic.
Carcinoma ex pleomorphic adenoma requires histology of both benign (pleomorphic adenoma precursor) and malignant components, with a transition zone between the two parts. The malignant component may appear as any of the other malignant epithelial lesions listed above (with adenocarcinoma NOS or MEC being the most common), or it may be undifferentiated.
Adenocarcinoma NOS exhibits cells with glandular or ductal differentiation but lacks distinctive features of other tumors and cannot be classified further.
Mucoepidermoid carcinoma has three cell types on examination: epidermoid-squamous cells, mucin-producing cells, and intermediate cells.
Ductal carcinoma resembles mammary ductal carcinoma and is composed of solid epithelial nests in an infiltrative, lobular, or cribriform arrangement and usually has a high mitotic rate and areas of necrosis.[10][14]
EMZL reveals diffusely-infiltrating small cells with abundant cytoplasm and slightly indented nuclei without nucleoli. Follicular lymphoma displays a partially follicular growth pattern with closely packed neoplastic cells without mantle zones. DLBCL manifests as diffusely-growing large B cells with a nucleus twice that of a normal lymphocyte and has many morphologic variants. Mantle cell lymphoma exhibits a monotonous pattern of small to medium lymphocytes with scant cytoplasm, irregular nuclei, and no nucleoli.
History and Physical
Demographic data is essential to collect, including age, gender, ethnicity, and race. Systemic medical history is also vital to ascertain possible etiologies: history of recent illness, history of autoimmune or inflammatory disease, history of cancer or chemotherapy and/or radiation, history of systemic immunosuppression, history of prior trauma or surgery. A comprehensive review of systems may pick up on systemic autoimmune or inflammatory disease associations.
Obtain a thorough ophthalmologic history regarding blurry vision, double vision, change in outward appearance or facial symmetry, pain, light sensitivity, pain with eye movement, eyelid swelling or redness, discharge, tenderness, tearing, dryness. Consider comparing old photographs to evaluate changes in eye or eyelid position or appearance.
A complete eye exam by a trained optometrist, ophthalmologist, or oculoplastic and orbital surgeon should be performed. This includes checking visual acuity for decreased vision, pupillary light reflexes for adequate responses and the presence of a relative afferent pupillary defect (rAPD), and other signs of optic neuropathy such as color vision deficits, red light saturation, and visual field defects.
Evaluating ocular motility can provide the degree of any limitations and the presence and pattern of diplopia. It is also prudent to assess any globe malposition, especially inferiorly and medially for lacrimal gland tumors, and perform exophthalmometry to evaluate proptosis. Digital palpation of the orbit and lacrimal gland may identify masses, and there may be resistance to retropulsion of the globe if a mass is present.
Palpation of regional lymph nodes can reveal lymphadenopathy. Hypoesthesia of the first or second divisions of the trigeminal nerve should be checked to evaluate for orbital nerve compression or invasion. There may be eyelid edema, erythema, or warmth and a directly visualized enlarged and tender lacrimal gland with secondary upper lid ptosis or S-shaped lid deformity. The conjunctiva may be injected, especially overlying the area of the lacrimal gland. An intraocular exam is also needed to evaluate for signs of uveitis.
Evaluation
It may be difficult to distinguish between benign and malignant lesions based on clinical evaluation alone. More rapid onset may hint towards a more aggressive or malignant process. If there was slow progression initially that then became rapid, it could indicate the malignant transformation of a pleomorphic adenoma into a carcinoma ex pleomorphic adenoma. The presence or absence of pain can also help predict the nature of the offending lesion, as the most common malignant lesion, adenoid cystic carcinoma, can exhibit perineural invasion. Usually, orbital imaging is helpful and often necessary to confirm and characterize a possible orbital lesion. Characteristics to discern are whether the tumor is solid vs. cystic, well-defined vs. infiltrative, the presence or absence and amount of calcification, the presence of fat or fluid, bony remodeling or destruction, and contrast enhancement.[15][16] Extension of a mass posteriorly towards the orbital apex ("wedge sign") is a finding that correlates with concerning disease and the need for biopsy.[17]
Computed tomography (CT) is expedient, widely available, cost-effective, and especially helpful for lacrimal fossa anatomy. It is widely considered the initial imaging modality of choice for lacrimal gland lesions, especially if a benign tumor is suspected. It can identify the presence and size of a mass and its effects on surrounding structures such as the bone (most importantly), extraocular muscles, and the globe. Contrast administration is also helpful to look for enhancing lesions. Computed tomography scans also readily show calcification within a lesion, which can help differentiate the offending mass.
Magnetic resonance imaging (MRI) is more expensive, harder to obtain, and has a longer duration but gives superior soft tissue detail. The various image processing sequences allow for detailed characterization of the mass in question, and DWI may delineate benign versus malignant.[18]
Unlike CT, MRI has limited bone visualization but can better evaluate tumor involvement of orbital nerves, important for adenoid cystic carcinoma.[16] Because of this, it may be a better imaging modality when a malignant lesion is suspected.
Many cases may require both CT and MRI imaging before further management decisions.
Treatment / Management
The management of malignant lacrimal gland tumors has not been clearly established because of their rarity and poor prognosis. Yet, the established goals for treatment are local tumor control and prevention of both local recurrences and distant metastases.[19]
The first step in all suspected malignancies is establishing a formal histological diagnosis, usually achieved via incisional biopsy. In some cases, small or easily accessible lesions can be excised entirely. Treatment can then be directed by tumor grading and staging. Historically the treatment of most lacrimal gland carcinomas has included orbital exenteration with or without bone removal and adjuvant high dose radiation 4 to 6 weeks after surgery.
Adenoid cystic carcinoma has had developments in treatment strategies, though there is still no widespread consensus. Treatment may involve a combination of tumor removal, radiation therapy, and orbital exenteration with varying survival rates.[20] There has been a push towards globe-sparing surgery due to the functional, cosmetic, and psychologic morbidity of orbital exenterations, with similar survival results.[21][22]
More recently, intra-arterial cytoreductive chemotherapy (IACC) has been used successfully before orbital exenteration and radiation therapy and may be combined with globe-sparing surgery and adjuvant radiation therapy.[23] Nonetheless, extensive surgery may still be indicated for locally advanced cases but does not prevent metastasis or improve survival.[24]
The treatment of carcinoma ex pleomorphic adenoma depends on the histologic grade and stage at presentation. An isolated, well-defined mass may be treated with excisional surgery alone without radiation. On the other hand, ill-defined lesions invading the tumor capsule or surrounding orbital tissue are often treated similar to ACC with excision, possible orbital exenteration, and adjuvant radiation therapy.[25]
Adenocarcinoma NOS and the other malignant lesions are usually treated similarly to ACC with exenteration and radiation therapy. If only a microscopic tumor is present on histopathology, plaque radiation therapy can be considered a treatment option for any lacrimal gland malignancy.[26]
The management of ocular adnexal lymphoma depends on the extent of systemic involvement. First, an incisional biopsy is necessary to establish the diagnosis and ascertain the subtype of lymphoma and its genetic characteristics. This is followed by a systemic workup by an oncologist, potentially including systemic imaging and/or a bone marrow biopsy. Unlike the other malignancies of the lacrimal gland, definitive treatment of lymphoma is not surgical. If the lymphoma is located in the orbit only, then external beam radiation therapy is the established initial treatment, except for DLBCL and mantle cell lymphoma, which are treated by chemotherapy.[18] Chemotherapy is indicated if there is systemic involvement, with possible radiation therapy as needed in select cases.
Differential Diagnosis
In addition to the malignant lesions above, other etiologies can present as lacrimal gland fossa lesions. Two major categories for these other causes are inflammatory lesions and benign tumors. Altogether, inflammatory lesions make up the most common cause of lacrimal gland enlargement.[5] The single most common etiology is dacryoadenitis, which can be unilateral or bilateral and inflammatory or infectious.
Inflammatory Lesions[2]
- Idiopathic orbital inflammatory syndrome (IOIS), or orbital pseudotumor
- IgG-4 related disease
- Sarcoidosis
- Granulomatosis with polyangiitis
- Dacryoadenitis
- Sjogren syndrome
- Thyroid eye disease
Benign Tumors[4][8]
- Epithelial
- Pleomorphic adenoma
- Dacryops (lacrimal gland cyst)
- Oncocytoma
- Myoepithelioma
- Cystadenoma
- Warthin tumor
- Lymphoproliferative
- Reactive lymphoid hyperplasia (benign lymphoepithelial lesion)
- Mesenchymal
- Capillary or cavernous hemangioma
- Angiolymphoid hyperplasia with eosinophilia
- Granular cell tumor
- Fibrous histiocytoma
- A solitary fibrous tumor (hemangiopericytoma)
- Neurofibroma
- Schwannoma
Some lesions can be found near the superolateral orbit and can be confused for lacrimal gland tumors. They include:
- Epidermoid or dermoid cyst, commonly found at the frontozygomatic suture and may have an intraorbital component
- Prolapsed orbital fat, causing fullness of the lateral upper lid or a mass in the superotemporal fornix
- Dermatolipoma, commonly seen under the superotemporal conjunctiva
- Prolapsed lacrimal gland, giving the appearance that it has been displaced by a mass
Lacrimal gland enlargement can also be seen in amyloidosis. Though rare, amyloid deposition in orbit, including the lacrimal gland, is usually due to a localized disease process rather than associated with systemic amyloidosis.[27]
Staging
Due to their common embryologic origin, lacrimal gland tumors are classified similarly to salivary tumors as dictated by the World Health Organization's guidelines.[28] Lacrimal gland tumor staging is according to the American Joint Committee on Cancer (AJCC), the eighth edition.[29] Lacrimal gland lymphomas are staged similar to other ocular adnexal lymphomas as per the AJCC.[30]
Prognosis
Since adenoid cystic carcinoma is the most common malignant tumor, it has the most prognostic data of the lacrimal gland carcinomas. Overall, the prognosis is poor, and ACC has often been referred to as the "slow killer" since recurrences and metastasis can occur years after treatment, with most patients dying within ten years of diagnosis. Several prognostic factors have been identified in the literature, including tumor size, histologic subtype, perineural invasion, and the stage at diagnosis.[31]
Tumor size is one of the more important factors, with a better prognosis for tumors that are less than 2.5 cm in greatest diameter.[24] Histologically, these tumors typically have multiple subtypes, but the predominance of the cribriform (Swiss cheese) pattern may be a positive prognostic factor, whereas a predominance of "basaloid" or solid pattern may be a negative factor.[32][33][34] The AJCC TNM stage does reasonably well with predicting outcomes of patients, with a worse prognosis for recurrence, metastasis, and death for tumors less than stage T3a than for one wish stage T3a or greater.
For carcinoma ex pleomorphic adenoma, the prognosis depends on the identity and behavior of the malignant component of the tumor that has spread beyond the capsule of the original tumor. If the tumor is non- or minimally invasive, the prognosis is excellent. Otherwise, most patients die within a few years due to intracranial spread and metastases to the lungs, chest, and bone.[3][10]
Mucoepidermoid carcinoma is organized into low, intermediate, and high-grade tumors. The low and intermediate tumors carry a good prognosis, but high-grade tumors have a poor survival rate.[3]
Ductal carcinoma is an aggressive neoplasm that usually presents as locally advanced disease that is difficult to control, with a mortality rate of over 40%. Metastases develop in more than 50% of cases.
The prognosis for lymphoma overall is good, though it depends on the extent of systemic involvement. For extranodal marginal zone lymphoma localized to just the orbit is very good, as it is usually sensitive to radiation therapy. Diffuse large B cell lymphoma has three subtypes, with the ABC subtype having the poorest prognosis.[8]
Complications
Complications of malignant tumors are mostly related to the treatment involved in eradicating the tumor and preventing recurrences. The tumors may compress orbital structures like the globe or optic nerve if the tumors are large. Malignant lesions may erode through the orbital roof to gain access to the intracranial vault, or in the case of adenoid cystic carcinoma, may spread via the perineural route to access the CNS where severe neurologic changes may manifest.
Surgical biopsy or excision carries risks, and complications are related to the surgery performed and the techniques utilized. For anterior or lateral orbitotomies, the main complications of note include orbital hemorrhage, postoperative orbital cellulitis, damage to the extraocular muscles, globe, or optic nerve, dryness from decreased lacrimal gland function, cerebral spinal fluid (CSF) leak, or recurrence of the tumor.
Radiation therapy can also have adverse effects, including surrounding tissue damage, dermatitis of the skin, atrophy of the bone and soft tissues, and ocular effects, including retinopathy and dry eye disease. Chemotherapy can lead to many side effects, the most concerning being immunosuppression and the risk of infection. Vigilant short- and long-term monitoring after treatment is crucial to managing these complications and monitoring for tumor recurrences.
Deterrence and Patient Education
The management of malignant tumors in an area such as the orbit can be quite complicated. A thorough discussion is necessary to inform the patient of the potential causes, treatments, adverse events, and prognosis of both the disease processes involved and the treatment options. Whenever cancer is being discussed as a diagnosis, it is critical to approach the patient and situation with compassion and empathy. At times this may necessitate multiple discussions so the patient can process the information appropriately and make an informed decision.
The risks, benefits, and alternatives of any treatment (or lack of treatment) should be thoroughly explained. The emotional and psychological impact of the diagnoses involved and the treatments (surgery, chemotherapy, radiation, long-term monitoring for recurrence, etc.) and especially any complications that arise may require multi-specialty attention from a primary care provider, oncologist, psychologist, or psychiatrist.
Pearls and Other Issues
When evaluating any patient with an orbital mass, keeping a broad differential diagnosis is vital. A thorough history and clinical exam are paramount for determining risk factors for various disease processes and guiding diagnostic workup and treatment acuity. Malignant lesions often carry a poor prognosis, and thus expedient workup and treatment are essential.
Inflammatory signs are not often seen with tumors and may indicate infectious or inflammatory dacryoadenitis. Pain or hypoesthesia is often an indicator of malignancy as most benign lesions are painless. Imaging is equally important in diagnosis and the decision-making process for treatment; it can also aid with surgical planning. The treatment of malignant tumors requires a multi-specialty approach with physicians experienced in managing these orbital tumors and their complications.
| |
Adenoid Cystic Carcinoma |
Orbital Lymphoma |
| Age of onset |
Middle Age |
Older Adults |
| Gender |
Male |
Female |
| Associated Pain |
Yes |
No |
| Duration of onset |
Acute |
Indolent |
| Histopathology |
Various
Unencapsulated
|
Monoclonal sheet
Unencapsulated
|
| Treatment |
Combination Therapy
Excision/Radiation/Exenteration
|
Radiation (Local)
Chemotherapy (Systemic)
|
Enhancing Healthcare Team Outcomes
From start to finish, the journey from clinical evaluation to work up, then diagnosis to management, and subsequent monitoring treatment response to managing complications requires a large, interprofessional team of administrators, staff, technicians, nurses, and doctors. The treatments for malignant tumors often involve multiple surgeries, radiation treatments, chemotherapy infusions, and countless office visits. The patient will interact with dozens of individuals in the professional setting during their care. Effective communication between all parties is necessary to promote the efficient use of medical resources and provide a smooth and effective experience for the patient. [Level 4]