Continuing Education Activity
The crystalline lens is thought to have the immune privilege, or the immune system has tolerance to the lens proteins. Exposure of the lens proteins to the rest of the ocular structures, whether due to trauma, advanced cataract, or retained cortical matter post-cataract surgery, causes ocular inflammation. The artificially implanted intraocular lens is made of inert material and does not usually cause inflammation by itself if it is placed inside the capsular bag. Lens-induced inflammation causes pain and redness in the eye. Immediate recognition of the condition with appropriate management is necessary to avoid severe ocular injury. The final management of lens-induced inflammation is usually surgical. Good final visual acuity can be achieved through prompt diagnosis and optimal management. This activity explores the etiology, pathophysiology, clinical signs and symptoms, differential diagnoses, and the management of lens-induced inflammation.
Objectives:
- Outline the etiology of lens-induced inflammation.
- Describe the clinical findings and investigations required for the evaluation of lens-induced inflammation.
- Review the available management options for lens-induced inflammation.
- Summarize the importance of patient education and the role of interprofessional teams to improve the outcomes of lens-induced inflammation.
Introduction
The term 'endophthalmitis phacoanaphylatica' was introduced by Verhoeff and Lemoine in 1922 when they reported patients who had increased inflammation (apparently sterile inflammation mimicking endophthalmitis) after they underwent extracapsular cataract surgery.[1] This disease entity was, however, recognized first by Straub in 1919. The immunological nature of this condition was proved when most patients reacted to an intracutaneous injection of lens proteins. Other names of such lens-induced inflammation (uveitis) include phacolytic glaucoma, phacogenic uveitis, phacotoxic uveitis, and phacoanaphylactic endophthalmitis. Lens-induced inflammation has an immune basis, but it does not involve immunoglobulin E or histamine (as seen in type I hypersensitivity reaction). Thus the term phacoanaphylactic endophthalmitis is not preferred now.[2][3]
The term phacotoxic uveitis is also avoided as lens protein has not proven toxic to the eye.[4] Exposure of the lens proteins to the immune system or alteration of immune tolerance to lens protein is supposed to cause lens-induced inflammation. Lens-induced inflammation occurs in various clinical scenarios, including leakage of lens proteins through an intact lens capsule in advanced cataracts (phacolytic uveitis or phacolytic glaucoma) and a broken anterior lens capsule in traumatic cataracts or after cataract surgery (phacoantigenic uveitis).
In modern-day cataract surgery, the retained cortical matter is not very commonly seen after cataract surgery. However, when there is residual cortex after cataract removal, with or without an intact posterior capsule, inflammation can occur. Such inflammation may present with ocular redness, pain, and sensitivity to light. Typically, lens-induced inflammation gets controlled after cataract surgery or removal of the retained lens matter. This review discusses the etiology, pathophysiology, clinical signs and symptoms, differential diagnoses, and the management of lens-induced inflammations.
Etiology
The causes of lens-induced inflammation include immune response to lens proteins following exposure due to conditions including:
- Hypermature or mature cataract with leakage of lens protein through an intact capsule,
- Incomplete removal of cataracts, and
- Trauma.
Most cases of lens-induced inflammation are reported in developing nations due to less access to medical facilities and delays in cataract surgery. The retained cortical matter was also more common with older surgical procedures. Improvement in microsurgical techniques has led to a lesser incidence of phacogenic uveitis.[5]
Chronic uveitis may still occur after modern surgery due to either retained cortex or nucleus pieces in the anterior segment, even after uncomplicated surgery where the posterior capsule is intact. Dislocation of the lens matter (partially or as nuclear pieces and/or cortical matter) into the vitreous cavity may occur due to posterior capsular rupture or zonular dehiscence and may induce an inflammatory reaction, the extent being determined by the size of the fragment, intraocular manipulations, patients' inflammatory response, and other comorbidities. In recent times, common causes of lens-induced uveitis after phacoemulsification include lens matter drop in the vitreous cavity or a small overlooked piece of nucleus or lens matter in the inferior angle of the anterior chamber. These complications are more common in eyes with small pupils, floppy iris, pseudoexfoliation, hard cataracts, and zonular weakness.[6]
Trauma causing rupture of the anterior capsule of the lens and exposure of lens proteins to the ocular tissues also causes an inflammatory reaction. Initially, uveitis may be unrecognized after trauma due to the presence of hyphema and corneal edema. Small perforation of the lens capsule may present later with a total cataract and severe inflammation. Cases of lens-induced uveitis have been reported in sympathetic ophthalmia, microphthalmia (persistent hyperplastic primary vitreous), after metastatic endophthalmitis in septic pneumonia, choroidal melanoma, in association with intraocular inflammatory mass, congenital cataracts, and after repeated intravitreal injections for diabetic macular edema in a patient with a history of surgery for congenital cataracts.[7][8][9]
The proposed mechanism in most such cases is a violation of the lens capsule resulting in exposure of the lens proteins. Though dropped lens (with an intact capsule) in the vitreous cavity usually does not incite inflammation (as noted in Marfan syndrome), lens-induced inflammation has also been reported in cases of posterior dislocation of the lens that did not have a compromise of the lens capsule.[10]
Epidemiology
In a retrospective analysis of patients presenting for cataract surgery to a tertiary care center, fifty patients had lens-induced glaucoma. They constituted 2.4 % of the 12,004 patients who underwent cataract surgery between 2005 and 2011. Thirty-nine (78%) of these patients had phacomorphic glaucoma, and 11 (22%) patients had phacolytic glaucoma. More than 40% of the patients had a best-corrected visual acuity of 20/200 or worse after cataract surgery.[11]
Postoperatively, retained lens material or nuclear fragments may cause lens-induced uveitis after cataract surgery.[6] Uveitis due to a retained lens fragment fifteen years after phacoemulsification was reported by Kang et al.[12]
Phacogenic uveitis is an uncommon occurrence due to improvements in cataract surgery techniques. The diagnosis of lens-induced uveitis is also often retrospective. Only six cases were clinically recognized in a retrospective analysis of 144 eyes diagnosed histopathologically as phacoanaphylactic endophthalmitis or lens-induced uveitis.[13] The lens-induced inflammation is a result of surgical or nonsurgical trauma. Inflammation due to trauma to the lens occurs anywhere between two days to fifty-nine years after the event. Histopathologically, there is zonal granulomatous inflammation surrounding the ruptured lens. The choroid was histopathologically involved in 76% of the cases in a study by Thach et al.[13]
Pathophysiology
The crystalline lens forms very early in fetal life. The development of the eye starts on day 22 of embryogenesis. Optic vesicles form as outpouchings from the forebrain around 25 days of gestation. They enlarge and come into contact with the surface ectoderm. The cells of the surface ectoderm overlying the optic vesicles form the lens placode. The lens pit appears inferior to the lens placode, deepens by cellular multiplication, and eventually separates from the surface ectoderm forming the lens vesicle. The primary lens fibers and the embryonic nucleus form around 40 days of embryonic life.[14]
The lens capsule is a basement membrane that develops from the lens epithelium. Secondary lens fibers form at seven weeks, and the fetal nucleus is formed at eight months. The lens proteins are sequestered from the other ocular structures histologically; the lens has a capsule, anterior epithelium made up of a single layer of cuboidal cells that elongate to form lens fibers at the equatorial region. The nucleus is the central part of the lens. As age increases, the lens consists of the embryonic nucleus (formed in the first three months of gestation), the fetal nucleus, which forms after that, the infantile nucleus, which develops from birth to puberty, and then the adult nucleus. The sequestration of lens proteins from the rest of the ocular structures and their unique antigenic properties form the basis for lens-induced inflammation when these proteins are exposed either through an intact or ruptured anterior capsule.[15]
The exact pathogenesis is unknown. Various hypotheses about the pathogenesis of lens-induced inflammation have been proposed:
- Loss of immune privilege of the crystalline lens and sequestration of lens proteins by the lens capsule (release of lens proteins resulting in a foreign body reaction),
- Loss or alteration of immune tolerance to lens proteins, and
- Possible role of anterior chamber-associated immune deviation (ACAID).
Traditionally, the crystalline lens has been considered immunologically privileged due to avascularity, absence of nerves, and isolation from fetal circulation.[2] Before the 1970s, it was believed that the lens proteins were sequestered from the immune system and were not recognized as self-proteins.[16] Thus, leakage of lens proteins was thought to cause a foreign body reaction or immune rejection resulting in lens-induced uveitis.[17] The soluble crystallins convert into insoluble ones with age, possibly increasing the autoantigenicity of lens proteins in mature or hypermature cataracts.[18][19]
However, later it was shown that lens proteins leak into the aqueous humor in normal conditions with an intact capsule of the clear crystalline lens.[20][21][22] In utero, the lens is surrounded by a highly vascular structure called tunica vasculosa lentis. Lens proteins are increased in the aqueous humor in eyes with cataracts. The immune system recognizes the lens proteins, as evidenced by the presence of lens protein-binding lymphocytes in the spleen of animals with normal lenses. These animals did not have a history of trauma or previous sensitization with lens proteins.[23] Some normal individuals show antibodies against lens proteins.[24]
Though foreign grafts are almost always rejected, lens-induced uveitis is relatively rare, considering the very common incidence of violation of the lens capsule. Also, the lens proteins are not species-specific or organ-specific.[24] The antigens of the lens have been detected in multiple ocular tissues (including the iris, retina, and choroid) and extraocular tissues (including the liver, basement membrane of the glomerulus of the kidney, brain, and serum).[25]
The immune response to lens proteins may vary in different species. The soluble lens proteins are weak antigens (alpha-crystallin and beta-crystallin) or lack antigenicity (gamma-crystallin). The difference in the immune response to lens proteins may be related to different alleles of major histocompatibility complex molecules.
It is currently agreed that lens-induced uveitis is a form of autoimmune response. Excess lens material in the anterior chamber may cause altered tolerance to lens proteins.[22] The inflammation is triggered by autoreactive T cells or Immunoglobulin G lens autoantibodies. ACAID protects the eye from damage caused by delayed-type hypersensitivity. The role played by ACAID in lens-induced uveitis is not yet clarified. Many factors influence the extent and type of immune reaction. The amount of retained lens matter in the anterior segment or posterior segment, surgical or non-surgical trauma, and the immune status of the patient have a role in the clinical picture and prognosis. Adhesion molecules on neutrophils (CD18) and adhesion molecules on the endothelium (ELAM-1, endothelial leukocyte adhesion molecule-1) have been shown to play an important role in the causation of experimental lens-induced uveitis (ELIU). Antibodies against these adhesion molecules significantly suppressed ocular inflammation in these animals with ELIU.[26]
As discussed previously, evidence of type I hypersensitivity (anaphylactic) reaction has not been reported in lens-induced inflammation. However, types II, III (immune complexes), and IV (delayed-type, cell-mediated) hypersensitivity reactions may play a role in the pathogenesis of lens-induced uveitis.[25] Recently, the concept of immune privilege of the eye and brain has been challenged with the suggestion of the presence of lymphatic drainage and immune surveillance promoting the immunoquiescence of these organs.[27][28][29][30]
A possible connection of the lens to the immune system may be via the zonules connecting it to the ciliary body.[30] It has also been shown that lens degeneration may activate an immune response involving multiple ocular tissues, including the retina, vitreous humor, and cornea.[30][29]
Histopathology
Granulomatous inflammation is seen on histopathology. Degenerated lens remnants are surrounded by polymorphonuclear leucocytes, epithelioid cells, macrophages, and multinucleated giant cells. Later, lymphocytes, eosinophils, and plasma cells are seen. Phagocytes are found around the lens capsule in cases of phacolytic glaucoma, with the trabecular meshwork getting clogged by macrophages. Iris is involved more than the choroid.[17]
Vasculitis may also be seen in the retina.[31] Before the advent of modern-day cataract surgery, many cases of lens-induced inflammation underwent enucleation due to advanced glaucoma and severe inflammation causing painful blind eyes.[32] The occurrence of non-granulomatous uveitis has also been reported.
History and Physical
Advanced cataracts and surgical or non-surgical trauma to the lens capsule can lead to lens-induced uveitis. Typically, the time lapse from trauma or surgery is within two weeks of the event.[33] Rarely, inflammation may be months or even years after the surgery due to retained lens fragments. Usually, the patients are elderly with a history of cataract surgery in one eye. Due to good vision in the operated eye, cataract surgery of the other eye is often delayed. Such patients come with sudden onset pain and redness in the non-operated eye. Usually, there is a history of poor vision and white pupillary reflex in the involved eye due to advanced cataracts. Phacolytic glaucoma was described by Gifford in 1900. The name was given by Flocks in 1955.[32]
Many factors may influence the presentation, including the composition of the lens, amount of antigen liberation, immunological pre-sensitization, and genetic and individual factors. Symptoms include pain, redness, photophobia, watering, and decreased vision. Retained lens matter in the vitreous cavity may also cause floaters. Cystoid macular edema may also cause a reduction in vision. Visual acuity may be the perception of light (PL) in advanced cataracts or cases of severe inflammation. It may also range from PL to 20/20 in cases of post-operative uveitis. Inflammation may be seen within a few hours after trauma or surgery or might be delayed.[34]
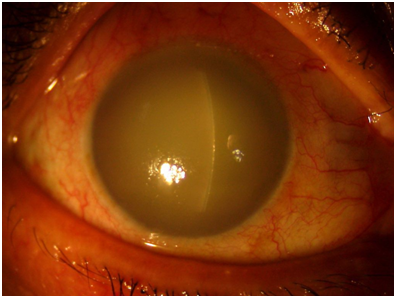
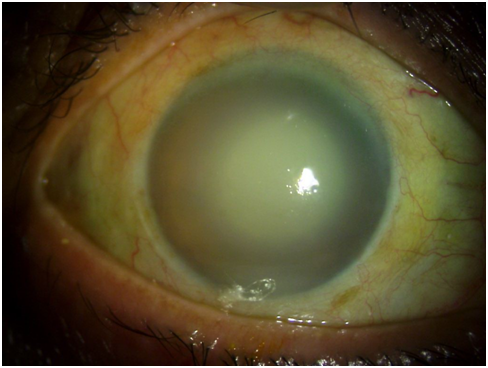
Typically, granulomatous anterior uveitis is noted though vitritis may also be present. Lid edema, circumciliary congestion or even diffuse congestion, corneal epithelial and stromal edema, presence of keratic precipitates (KPs, mutton fat or fine KPs), anterior chamber cells, marked flare, lens matter in the anterior chamber, hypopyon, peripheral anterior synechiae, posterior synechiae, rubeosis iridis, pupillary membrane, and iris nodules may be seen. Intraocular pressure (IOP) is often high.[34]
Causes of high IOP include trabeculitis, phacolytic glaucoma (altered lens protein and macrophages blocking the angle of the anterior chamber), and peripheral anterior synechia. The underlying cataract is usually advanced, and there may be wrinkling or calcification of the anterior capsule. The posterior segment may not be visible due to dense cataracts. Lens fragments, vitreous cells, intense vitritis, traction bands, vasculitis, cystoid macular edema, and epiretinal membrane formation may occur. Advanced cases may present with a picture very similar to endophthalmitis. Untreated cases may result in phthisis or a painful blind eye due to absolute glaucoma. Hypotony may result from choroidal effusion, ciliary shutdown, or cyclitic membranes.
Evaluation
Slit-lamp photographs or anterior segment photographs using a fundus camera help in documenting the disease, explaining the patient, and monitoring the effect of therapy.
Gonioscopy is important to document peripheral anterior synechiae, goniosynechiae, new vessels, lens matter, and retained nucleus fragments. However, in the acute stage, the corneal edema may not allow good visualization of the angle of the anterior chamber.
Ultrasound biomicroscopy is a useful modality to visualize the angles of the anterior chamber in hazy corneas.[35]
Anterior segment optical coherence tomography and ultrasonic biomicroscopy can be used as diagnostic modalities in uveitis glaucoma hyphema syndrome to detect malposition of the intraocular lens, iris adherence to the peripheral cornea and can detect the cause for persistent inflammation after cataract surgery.[36][37]
Lens-induced uveitis is often missed clinically.[38] If an anterior chamber tap is done, giant macrophages may be seen, which are full of lens material. Anterior chamber tap may help to differentiate between lens-induced uveitis and endophthalmitis.[39] Histopathology shows zonal granulomatous inflammation.
Ultrasound B scan is important in cases of advanced cataracts to look for an intraocular foreign body in cases of perforating injury, vitreous echoes, dropped lens matter, traction bands, and optic nerve head cupping in cases of phacolytic glaucoma. Ultrasound also helps rule out endophthalmitis, panuveitis, retinal detachment, and intravitreal cysticercus.[38][31]
Optical coherence tomography of the macula is especially useful in patients with uveitis after cataract surgery to rule out cystoid macular edema. It may also help in ruling out other differential diagnoses.[40]
Treatment / Management
Lens-induced inflammation has to be recognized early and managed promptly. Intraocular pressure measurement by Goldmann applanation tonometer should be done. Gonioscopy (if the media allows) and ultrasound B-scan should be done.[41] The definitive management of lens-induced inflammation in an eye with a cataract is cataract extraction.
In lens-induced uveitis after trauma, calculating the power of the intraocular lens (IOL) may be challenging. In an eye with a disfigured cornea, the keratometry of the other eye may have to be used for the calculation.[42] Axial length measurement of the eye with trauma if the globe is intact (blunt trauma or repaired corneoscleral perforation) can be done to calculate intraocular lens power. In the case of the eye with phacolytic glaucoma or phacogenic uveitis, biometry of the same eye can be done. Preoperatively, reduction of intraocular pressure should be tried with intravenous mannitol, oral acetazolamide, and topical glaucoma medications. Topical steroids help quieten the eye and may reduce postoperative ocular inflammation in an advanced cataract with lens-induced inflammation. The formation of arachidonic acid is blocked by corticosteroids, thus preventing the release of inflammatory mediators such as prostaglandins, leukotrienes, and thromboxane.[43]
If needed, topical steroids may be given hourly. In cases of severe inflammation, systemic steroids may be necessary. Topical antibiotics are also started preoperatively. Cycloplegia is extremely important to break posterior synechiae, stabilize the blood-aqueous barrier, relieve ciliary spasms and decrease the pain.[41]
Lens matter aspiration in a young patient may be done by manual techniques or by irrigation aspiration probe, depending on the facilities available. In older patients, phacoemulsification, manual small incision cataract surgery (MSICS), or extracapsular cataract extraction (ECCE) can be planned based on the surgeon's comfort. Special care should be taken in traumatic cases, as preexisting damage to the posterior capsule of the lens may be present. Adequate anterior vitrectomy should be performed in such cases before implanting the IOL if there is intraoperative vitreous prolapse. The wounds and anterior chamber should be free from vitreous after surgery.
In eyes with lens-induced inflammation associated with advanced cataracts, zonular compromise or poor pupil dilation may be encountered. Also, the corneal endothelium may be compromised in such cases resulting in delayed clearance of the postoperative corneal edema.[41] Intraoperatively, the complete removal of lens matter is important. In cases of advanced cataracts causing phacolytic glaucoma or phacogenic uveitis, thorough cortex and lens fragments from the anterior chamber should be removed. However, many such eyes with advanced cataracts have scarring of the anterior or posterior capsule of the lens and very adherent corticocapsular adhesions. Excessive surgical manipulation of such areas may cause further ocular damage. The corneal haze may compromise the intraoperative view during cataract surgery.[44][45]
Intracameral triamcinolone may reduce postoperative inflammation and help identify vitreous in the anterior chamber in cases with posterior capsular rent.[46] However, it may cause a postoperative rise in intraocular pressure. A posterior chamber intraocular lens in the bag is a preferred option. In retained nucleus fragments in the angle or retained cortical matter with the intact posterior capsule after cataract surgery, anterior chamber wash can easily be performed.[47]
In the scenario that the posterior capsule is ruptured, the pars plana route may be used by the vitreoretinal surgeon to remove the nucleus fragments and lens matter from the vitreous cavity. Small lens matters in the vitreous cavity can be observed, which are usually absorbed with time.
Differential Diagnosis
Lens-induced uveitis has to be distinguished from several other causes of uveitis which may be infective or noninfective. In the elderly, endogenous endophthalmitis may occur due to systemic illness and lowered immunity. A history of hospitalization, intravenous drug use, or intravenous infusion should be asked. General examination and blood work for liver, kidney, and lung diseases may rule out this condition. Urinary tract infection should be ruled out.
In cases of trauma, bacterial endophthalmitis or fungal endophthalmitis may occur.[39] Hypopyon may be present in both conditions.[48] Ultrasound B scan shows vitreous opacities in both these conditions. History of injury with contaminated tools may also point toward an infective etiology. Microbiological work-up of aqueous and /or vitreous tap may be done. Clinical clues toward a diagnosis of endophthalmitis include severe pain, severe redness, severe photophobia, mucopurulent ocular discharge, severe vision loss (including no PL or inaccurate projection of rays), and lid edema.
Propionibacterium acnes can cause chronic postoperative endophthalmitis months after cataract surgery. Granulomatous uveitis with hypopyon, plaques in the posterior capsule, and vitritis are the presenting features. Infectious postoperative uveitis can also be caused by Staphylococcus epidermis and candida.[49][50]
Sympathetic ophthalmia may have an association with phacogenic uveitis. Choroidal thickening is more common in sympathetic ophthalmia. Both eyes are involved in sympathetic ophthalmia.[51] The presence of multifocal areas of subretinal fluid or disc hyperemia in the fellow eye points towards the diagnosis. The fundus fluorescein angiogram and optical coherence tomography of the retina play a crucial role in diagnosing sympathetic ophthalmia. Chan has described phacoanaphylactic endophthalmitis, sympathetic ophthalmia, and Vogt-Koyanagi-Harada (VKH) disease as a spectrum of uveitis. There is a history of trauma in sympathetic ophthalmia, leakage of lens proteins with or without rupture of the lens capsule in phacoanaphylactic endophthalmitis, and skin, ear, and central nervous system are involved in VKH. Bilaterality is a feature of both sympathetic ophthalmia and VKH.[52]
Uveitis due to collagen vascular disorders such as ankylosing spondylosis and sarcoidosis should be ruled out by investigations for these conditions and by history taking.
Prognosis
A good visual outcome is usually achieved with early diagnosis and prompt treatment. Untreated, long-standing inflammation has a poor prognosis for many reasons, including corneal edema, endothelial damage due to uveitis, rubeosis iridis, glaucoma, cystoid macular edema, vitreous traction bands, retinal vasculitis, and even phthisis.[53]
Advanced cataracts may be challenging to operate by phacoemulsification due to the hardness of the nucleus, lax zonules, and friable capsules of the lens. Secondary glaucoma may persist after cataract extraction needing medical and sometimes surgical management.[54]
Complications
The occurrence of lens-induced inflammation and secondary glaucoma may lead to less than satisfactory outcomes after surgery. Postoperative inflammation, secondary glaucoma, and cystoid macular edema are more common. Retinal vasculitis may be the result of severe intraocular inflammation. In addition, rubeosis iridis can be present preoperatively, leading to hyphema post-operatively. Severe complications include absolute glaucoma or phthisis.[55]
Postoperative and Rehabilitation Care
Most cases of lens-induced inflammation undergo cataract extraction with good postoperative outcomes—preoperative antiglaucoma medications and topical and systemic steroids aid in controlling intraocular pressure and inflammation. Complete removal of lens matter during surgery is important. Postoperative use of topical steroids and antiglaucoma medications may be needed for a longer time than routine cataract surgery. Posterior segment evaluation is mandatory after surgery as preoperatively, and the media is hazy due to dense cataracts.[41] In patients with comorbidities such as diabetes and hypertension, extra care needs to be taken if oral steroids are given. In post-traumatic cataract surgery, attention needs to be given to intraocular pressure monitoring, posterior segment evaluation, and examining the corneoscleral sutures in perforating injuries.[56]
Deterrence and Patient Education
Lens-induced inflammation is more likely to occur in an aging population and in areas where access to health care is difficult. The importance of regular health check-ups and ocular examinations by holding eye camps in underprivileged areas help to detect cataracts in the early stages and prevent the occurrence of phacolytic glaucoma and phacogenic uveitis. Among older generations, waiting for cataracts to attain "maturity" is still the norm.
Educating the patient population through mass media and sending healthcare personnel to rural areas for screening the adult population are some of the means to prevent lens-induced inflammation from occurring. On the other hand, traumatic cataracts can occur in both developed and developing nations. Prevention of industrial accidents by wearing protective gear during hazardous activities, recognizing damage to the lens capsule, and managing traumatic cataracts with early surgical intervention may help save vision.[56]
Pearls and Other Issues
Diagnosis of lens-induced uveitis is primarily clinical. A hypermature cataract may leak proteins through an intact lens capsule. This leads to lens proteins inciting an inflammatory response causing phacogenic uveitis and secondary glaucoma. In traumatic cataracts after a penetrating injury, the anterior and sometimes even the posterior capsule may be compromised, leading to exposure of cortical material, which may incite an inflammatory response. After cataract surgery, retained lens material (nucleus fragments or cortex) can cause inflammation for days, months, or even years after surgery.[57]
It is important to elicit a proper history, do a thorough slit lamp evaluation, measure intraocular pressure, perform a gonioscopy and do an ultrasound B scan to obtain the correct diagnosis. Management involves controlling the inflammation with topical and, if necessary, systemic steroids, control of IOP and cataract surgery, or removal of retained lens material. Early diagnosis and appropriate treatment can save the eye and restore vision. During the height of the COVID pandemic, many patients had delayed seeking medical help and care. Cataract surgery is an elective procedure and has been postponed by many patients leading to an increase in the maturity of the lens and an increased incidence of lens-induced inflammations.[58]
Enhancing Healthcare Team Outcomes
Most patients with advanced cataracts who have phacogenic uveitis are elderly with co-morbidities. They need physician review to control diabetes, hypertension, and other systemic disorders. High intraocular pressure due to phacolytic glaucoma has to be controlled by antiglaucoma medications, including systemic acetazolamide or intravenous mannitol, which needs monitoring by the nursing staff. Options for anesthesia in cataract surgery include topical proparacaine, peribulbar lignocaine, sub-Tenon lignocaine, intracameral lignocaine, sedation, and general anesthesia.[59] Ocular inflammation due to lens-induced glaucoma causes severe ocular discomfort and pain. Perioperative pain relief and appropriate patient monitoring during surgery may need to be managed by an anesthetist team. Traumatic cataracts may happen more at a younger age. Cataract surgery is performed under general anesthesia with anesthetic agents that lower intraocular pressure.[60] Management of phacogenic uveitis needs teamwork with Ophthalmologists, nursing staff, and anesthetists. Optometrists and technicians help calculate the power of the intraocular lens accurately in the scenario of hazy corneas or corneas with sutures and uncooperative patients who are in severe pain due to inflammation and high IOP.