Continuing Education Activity
This activity focuses on the prescription of aducanumab, a groundbreaking amyloid ß-directed monoclonal antibody for the treatment of Alzheimer disease (AD). Granted accelerated approval by the United States Food and Drug Administration (FDA) in June 2021, aducanumab is the first AD treatment to receive formal regulatory clearance since 2003. The approval was based on clinical trial data demonstrating a reduction in amyloid plaques in treated individuals.
This educational activity discusses key aspects such as indications, patient selection strategies, the mechanism of action, adverse event profiles, mandatory monitoring protocols, and contraindications for aducanumab use in AD management. The course emphasizes the pivotal role of interdisciplinary care teams in navigating these factors to deliver comprehensive care to individuals with AD, underlining the significance of close monitoring for potential adverse effects, notably amyloid-related imaging abnormalities (ARIA), which may necessitate dosing interruptions.
Objectives:
Differentiate the mechanism of action of aducanumab, especially in comparison to cholinesterase inhibitors and the NMDA-receptor antagonist, memantine.
Select patients carefully and in accordance with criteria for appropriate use and guidelines for prescribing and monitoring.
Identify the possible side effects of aducanumab, including criteria for discontinuation.
Collaborate with all members of the interprofessional team including primary care practitioners, neurologists, geriatric psychiatrists, geriatricians, neuroradiologists, pharmacists, and emergency department personnel to provide efficient, comprehensive, and coordinated care, plus capturing and responding to amyloid-related imaging abnormalities.
Indications
Note: In late January, 2024 Biogen announced the discontinuation of aducanumab, citing a "reprioritazation" of the company's priorities in the AD space. Biogen explicitly disavowed any concerns about safety or efficacy underlying this decision.
Alzheimer disease is a progressive, chronic, neurodegenerative disease and the most common etiology of dementia.[1] In dementia patients aged 65 and older, Alzheimer disease accounts for two-thirds of the dementia cases, and it is the sixth most common cause of older adult mortality in the United States.[2] Alzheimer disease's characteristic hallmarks are extracellular neuritic plaques generated by the deposition of beta-amyloid protein and intracellular buildup of tau proteins, resulting in intracytoplasmic neurofibrillary tangles(NFT).[3][4][5]
Disease onset is insidious, causing a gradual decline of behavioral and cognitive abilities that manifest as an initial short-term memory impairment accompanied eventually by impaired language, attention, comprehension, and executive functioning.[1] Current symptomatic management of Alzheimer disease entails treatment with cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) antagonists, as there is no cure or curative treatment to date.[1][6] The presence of 1 or 2 APOE ε4 alleles increases the risk for AD development.
A diagnosis of AD with MCI or the mild stage of AD dementia must be confirmed before considering treatment with aducanumab. A thorough history and physical and neurological examination of the patient should be conducted, incorporating a timeline of the onset and progression of cognitive decline.[7] Underlying co-occurring disorders that may contribute to cognitive deterioration should be assessed thoroughly by laboratory workup: complete blood count (CBC), comprehensive metabolic panel (CMP), thyroid function tests, hormone (TSH and free T4), lipid panel, liver function tests (LFT), and vitamin B12 concentrations.
Brain imaging to rule out potential neurological conditions such as normal pressure hydrocephalus (NPH), vascular dementia, malignancy, or subdural hematoma should also be undertaken as part of the dementia diagnostic workup.[7].
FDA Conditional Approval Initially Granted in July 2021 under the Program for Accelerated Approval
Biogen first presented positive results from its phase 1b study of aducanumab in early 2015; they enrolled the first subject in a global phase 3 clinical trial approximately 6 months later. In autumn 2019, Biogen announced plans for a regulatory filing; the Biologics License Application (BLA) was submitted to the FDA in July 2020 after delays caused by the early stages of the coronavirus pandemic.
Eventually, Biogen completed 2 phase 3 clinical trials with identical designs. Known individually as EMERGE and ENGAGE, both trials were initially terminated on grounds of futility before they were reinstated after additional blinded data became available. Based on this additional data, the researchers determined that EMERGE had met its primary outcome measure (the CDR "sum of boxes"); ENGAGE did not meet its primary outcome, but "benefits were observed" in study participants on higher doses for more extended periods. Knopman et al analyzed the data from 2 clinical trials with aducanumab. The investigators concluded that the efficacy of aducanumab cannot be proven when the 2 clinical trials yield different results.[8]
In November 2020, the FDA's Peripheral and Central Nervous System Drugs Advisory Committee met to discuss the aducanumab BLA. A series of 4 votes culminated in the near-unanimous (there was a single abstention) verdict that the advisors did not find reasonable grounds to consider the results of the clinical trials as evidence of aducanumab's effectiveness for treating AD. Within days of the July 2021 accelerated approval decision, 3 members of the Advisory Committee, comprising one-third of its nongovernment members, resigned, citing "serious potential to impair future research" and deeming the FDA's ruling "the worst approval decision that FDA has made....."[9]
In July 2021, the FDA granted accelerated approval for aducanumab; the decision was not based on the clinical data but rather on an intermediate outcome, namely the drug's ability to reduce the burden of excess β-amyloid in the brain and the attendant "reasonable" likelihood that amyloid reduction would benefit patients.[10] In conjunction with the approval, the FDA required that Biogen conduct a phase 4 clinical trial through 2030 to evaluate risks and benefits over the long term. Enrollment in the phase 4 trial was not a requirement for prescribing aducanumab.
FDA Approved Indication
Aducanumab is approved to treat Alzheimer disease in patients who have MCI-AD or who are in the mild dementia stage of the disease.[11][12][11] The first iteration of the package insert described the indication simply as "Alzheimer disease," but the indication was given greater specificity within days of the accelerated approval.[7] Aducanumab has not been studied to date in individuals without cognitive impairment or in those who have progressed beyond the mild stage of the disease. Aducanumab's continued use and full approval will require further verification of its clinical benefits in post-approval studies.
Non-FDA Approved Indication
At present, there are no known off-label indications or uses of aducanumab. A month after the accelerated FDA approval for aducanumab, explicit concerns about its off-label use in the setting of cerebral amyloid angiopathy (CAA) were raised by the leaders of the International CAA Association, who underscored substantial uncertainties and concerns about both the safety and efficacy of aducanumab in patients diagnosed with CAA.[13]
Appropriate Use Criteria for the Prescription of Aducanumab
Appropriate use recommendations (AUR) for aducanumab were crafted by an expert panel and published in July 2021 to complement the information in the package insert.[14] The authors outlined the clinical resources they deemed essential for the safe use of aducanumab, recommending:
- Clinicians skilled in the detection and recognition of prodromal and early-stage AD
- Access to imaging for brain MRI (initial and monitoring) and amyloid PET scans or individuals experienced in performing LPs
- infusion resources, whether in-office, a general-use infusion center, an AD-specific infusion suite, or home infusion with a visiting nurse
- Neuroradiologists who are proficient in the recognition of amyloid-induced imaging abnormalities (ARIA) on brain MRI
- Clinicians who are knowledgeable about the clinical recognition and management of ARIA
- Clinicians and staff who can deliver culturally competent care
- Availability and use of resources for patient and family education
- Genetic counseling for patients with questions about the implications of APOE genotyping and its interpretation
Recommended criteria for aducanumab eligibility included:
- Cognitive screening scores within a defined range, eg, MMSE 21-30 or MoCA 17-30
- Evidence of excess amyloid on amyloid PET scan or cerebrospinal fluid
- Stable cardiovascular, medical, or psychiatric disease
- Informed consent should be obtained for each patient
- Exclusion of the following individuals:
- those whose baseline MRI showed evidence of any hemorrhage (including cerebral microbleeds), superficial siderosis, stroke, or diffuse leukoaraiosis
- patients on anticoagulants
- pregnant women
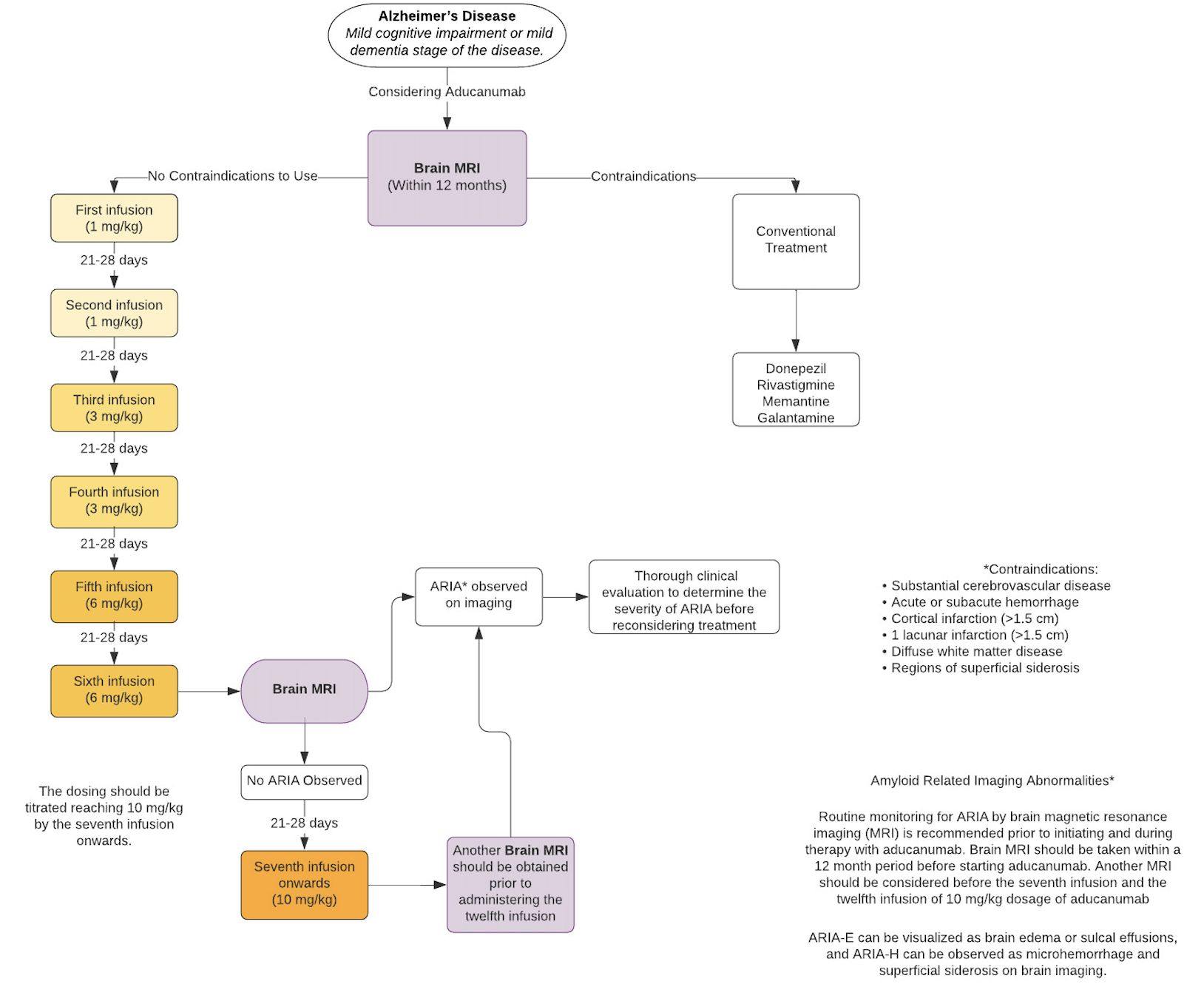
In 2022, the AUR recommendations were updated to ensure appropriate patient selection while improving shared decision-making, safety monitoring, and risk mitigation in treated patients.[15] The updated AUR explicitly underscored the importance of detecting past medical conditions that might predispose to ARIA or increase the likelihood of ARIA complications; the specified conditions included autoimmune or inflammatory conditions, seizures, or disorders associated with extensive white matter pathology. In addition, the updated AUR elevated the APOE recommendation from assuring the availability of genetic counseling to performing genotyping, given the known ε4 allele gene-dose effect and strong associations with ARIA, to better inform patient care decisions, discussions regarding risk, and clinician vigilance concerning ARIA. Specific recommendations were also made for the minimum amount of MRI monitoring, suggested before the 5th, 7th, 9th, and 12th infusions to improve ARIA detection. Finally, the expert panel outlined a lengthy list of signs and symptoms that should prompt the acquisition of an out-of-sequence MRI, including the acute or subacute emergence of focal neurological signs or symptoms, headache, confusion or disorientation (eg, delirium), dizziness or vertigo, nausea or vomiting, fatigue, blurred vision, other visual disturbance or impairment, new gait dysfunction, or seizure.
Biogen Announces Discontinuation of Aducanumab
In late January, 2024 Biogen announced the discontinuation of aducanumab, citing a "reprioritazation" of the company's priorities in the AD space. Biogen explicitly disavowed any concerns about safety or efficacy underlying this decision.
Mechanism of Action
Within the neuritic plaques that characterize Alzheimer disease neuropathologically, β-amyloid exists in several forms, eg, as monomers, oligomers, protofibrils, and fibrils. Oligomers and protofibrils are neurotoxic. Therefore, removal of them by monoclonal antibody therapy is thought to be helpful in the treatment of Alzheimer disease. Soderberg et al characterized the binding properties of aducanumab, gantenerumab, and lecanemab on several forms of amyloid-beta via immunodepletion, inhibition ELISA, and surface plasmon resonance studies.[16] Aducanumab, gantenerumab, and lecanemab bind to monomer amyloid-beta with low affinity. Lecanemab had 10-fold greater binding to amyloid protofibrils when compared to fibrils. Aducanumab and gantenerumab are bound preferably to β-amyloid fibrils over protofibrils. Soderberg et al concluded that these monoclonal antibody binding differences may be important for therapeutic efficacy and adverse effects.
Aducanumab is a high-affinity, fully human IgG1 monoclonal antibody directed against a conformational epitope found on aggregated soluble oligomers and insoluble fibrils of β-amyloid, one of the proteinopathies that cause AD.[17][18]
Biochemical and structural analyses showed that the aducanumab binds to a linear epitope formed by β-amyloid amino acids 3 to 7.[19] Compared to antibodies studied previously, aducanumab binds to the N-terminus of β-amyloid in an extended conformation. Based on weak monovalent affinity, fast binding kinetics, and strong avidity for epitope-rich aggregates, aducanumab has been shown to discriminate between β-amyloid monomers and oligomeric or fibrillar aggregates.[19]
The selectivity of aducanumab for aggregated β-amyloid forms results in reducing the brain burden of β-amyloid plaques.[17][20] In a small subset of patients using tau PET, a reduction in hyper-phosphorylated tau was also observed in the cerebrospinal fluid and in medial temporal neurofibrillary tangles.[21]
Administration
Aducanumab is a clear-to-opalescent and colorless-to-yellow solution currently available in single-dose vials for intravenous (IV) infusion administration. Based on the patient's body weight per kilogram (kg), aducanumab should be diluted with 100 mL of 0.9% sodium chloride injection before being administered as an infusion. The diluted agent solution be settled at room temperature before starting therapy and should be administered without delays. Unused diluted portions after administration of aducanumab should be discarded.
Available Single Dosages Vials
- 170 mg/1.7 mL (100 mg/mL)
- 300 mg/3 mL (100 mg/mL)
The dosing should be titrated, reaching 10 mg/kg by the seventh infusion. The IV infusion should be administered every 4 weeks with a minimum of 21 days between infusions. The infusion should be given over a 1-hour time frame.
Dosing Schedule
The regimen for aducanumab is titrated upward over time[22] according to the following titration schedule:
- First infusion (1 mg/kg)
- Second infusion (1 mg/kg)
- Third infusion (3 mg/kg)
- Fourth infusion (3 mg/kg)
- Fifth infusion (6 mg/kg)
- Sixth infusion (6 mg/kg)
- Seventh infusion onwards (10 mg/kg)
Missed dose analyses have yet to be performed. Patients who have missed an infusion of aducanumab are recommended to continue the next dose immediately at the dosage given at the previous infusion. For patients who have missed 3 or more infusions and are expected to continue treatment with aducanumab, the infusion should be re-initiated at a dose one step lower than the one administered previously. Continued treatment following the dosing titration schedule is recommended.[7]
See Image. Aducanumab Flow Chart.[7]
Adverse Effects
Adverse effects reported in EMERGE and ENGAGE, according to the package insert
- ARIA-edema (ARIA-E) (35%),[22] compared to 3% in the placebo arm
- ARIA-hemosiderin deposition (ARIA-H) microhemorrhage (19%), compared to 7% in the placebo arm
- ARIA-H superficial siderosis (15%), compared to 2% in the placebo arm
- Headache (21%), compared to 16% in the placebo arm
- Fall (15%), compared to 12% in the placebo arm
- Diarrhea (9%), compared to 7% in the placebo arm
- Confusion/delirium/altered mental status/disorientation (8%), compared to 4% in the placebo arm
- Immunogenicity (<1%)[23]
Aducanumab can cause a small elevation in serum aminotransferase; however, there are no reports of liver injury.[11]
Contraindications
The package insert for aducanumab defines no contraindications for its use in patients with MCI-AD or in patients with mild-stage AD.
No drug-drug interactions are currently reported as of this writing. Conventional therapy for Alzheimer disease was used during pivotal trials, and aducanumab may be used concomitantly with the other medications utilized to manage AD.[7][15]
Monitoring
Routine monitoring for ARIA by brain magnetic resonance imaging (MRI) is necessary, both before initiating the infusions and during ongoing aducanumab therapy.
A brain MRI must be obtained within 12 months preceding initiation of aducanumab. The package insert recommends monitoring with brain MRI prior to the 5th infusion (the first dose at 6 mg/kg), the 7th infusion (the first dose at 10 mg/kg), and the 12th infusion (the sixth dose of 10 mg/kg). An MRI should be obtained emergently in the case of a patient reporting any symptoms suggestive of either ARIA-E or ARIA-H. Note that monitoring was more stringent during the clinical trials, with the acquisition of a total of 9 MRIs between the first and the 12th infusions.
Dosing adjustments in renal and hepatic dysfunction were not studied during the clinical trials. Aducanumab is not expected to undergo hepatic metabolism or renal excretion. Immunogenicity of aducanumab was observed during clinical studies. Anti-aducanumab antibodies developed in 0.6% of subjects receiving aducanumab.
To date, no adequate study of aducanumab use during pregnancy and lactation in humans has been performed. Aducanumab was tested during animal reproductive studies, and no adverse effects were detected, although β-amyloid is not present in rat.[24]
Toxicity
Warning and Precautions
It is vital to obtain a brain MRI before starting aducanumab, as ARIA may develop with treatment. In cases where baseline MRI showed evidence of hemorrhage (including cerebral microbleeds), superficial siderosis, stroke, or diffuse leukoaraiosis, treatment with aducanumab should not be offered.
During the placebo-controlled period of the clinical trials, ARIA occurred in just over 41% of participants, with ARIA-E arising in 35% and ARIA-H occurring in fewer than 20%. ARIA-E can be visualized as brain edema or sulcal effusions, and ARIA-H can be observed as microhemorrhage and superficial siderosis on MRI. Symptoms can include but are not limited to headache, confusion, delirium, altered mental status, disorientation, dizziness, vision abnormality, and nausea. When data from the two clinical trials were combined into an integrated safety data set, ARIA-E occurred in just over 35% of patients receiving the 10 mg/kg dose; 26% of these patients experienced symptoms, most commonly headache, which occurred in 46%.[22] Most ARIA resolves without the need to discontinue treatment. Recurrent cases of ARIA-E may necessitate re-evaluation, and permanent discontinuation is recommended should a third episode of ARIA-E occur. Severe manifestations of ARIA require immediate and permanent cessation of therapy, with likely hospital admission and care for by clinicians—such as vascular neurologists—experienced in the sequelae of ARIA.
These recommendations may be updated as the real-world experience with aducanumab accumulates.
Hypersensitivity
Hypersensitivity reactions (eg, angioedema, urticaria) may occur during an aducanumab infusion. In such an occurrence, the infusion must be stopped promptly, and appropriate management should be initiated.
Enhancing Healthcare Team Outcomes
Aducanumab was fast-tracked to FDA approval in 2021 and is indicated for use in patients with MCI-AD or the mild dementia stage of AD. The care of patients with AD requires management and supervision from an interprofessional healthcare team. The healthcare team should include a neurologist experienced in the diagnosis and management of dementia, working in close collaboration with the patient's primary care clinician and with access to vascular neurology and neuroradiologists experienced in evaluating amyloid PET and recognizing ARIA. Social work and pharmacy can provide useful support. Routine care and follow-up for patients with AD by the interprofessional healthcare team promote efficient clinical evaluations and facilitate precise management plans and more reliable patient outcomes.
Infusion suite staff should be familiar with the particular needs of patients with cognitive impairment; elopements were reported during some of the clinical trials in monoclonal antibodies. Infusion suites should be prepared with training and equipment to manage hypersensitivity reactions, should they occur.
All members of the treating team must be aware of the potential adverse effects of ARIA and its asymptomatic or clinical manifestations. The prescribing neurologist should obtain informed consent and should fully advise patients of the warning signs of ARIA and the need for close clinical monitoring by brain MRI. The interprofessional healthcare team should routinely follow up with patients receiving aducanumab and thoroughly inquire about any new-onset symptoms indicative of ARIA. Such symptoms can include headache, confusion, delirium, altered mental status, disorientation, dizziness, vision abnormality, and nausea.