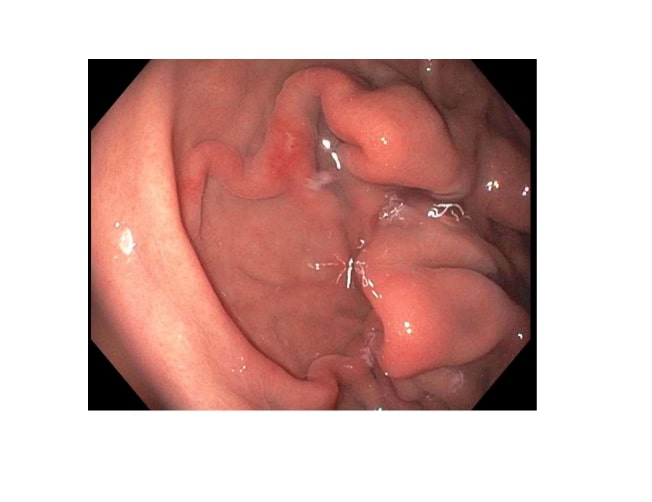
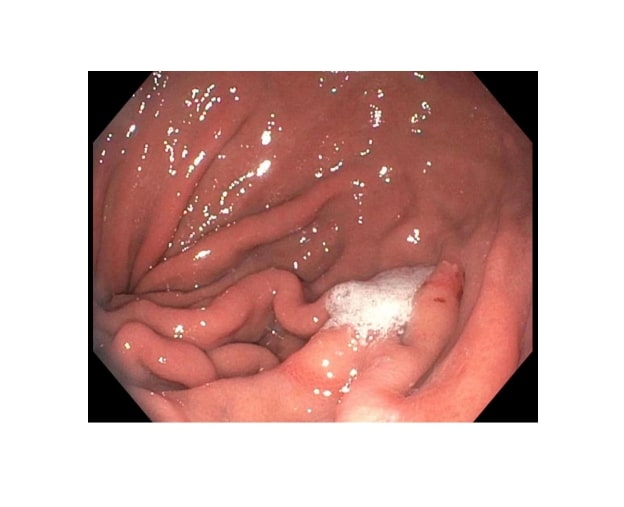
[1]
Maganty K,Smith RL, Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;
[PubMed PMID: 18622137]
[2]
Cameron AJ,Higgins JA, Linear gastric erosion. A lesion associated with large diaphragmatic hernia and chronic blood loss anemia. Gastroenterology. 1986 Aug;
[PubMed PMID: 3487479]
[3]
Moskovitz M,Fadden R,Min T,Jansma D,Gavaler J, Large hiatal hernias, anemia, and linear gastric erosion: studies of etiology and medical therapy. The American journal of gastroenterology. 1992 May;
[PubMed PMID: 1595651]
[4]
Richter IA,Rabin MS, The 'riding' ulcer: A report of 3 cases. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1979 Oct 6;
[PubMed PMID: 550413]
Level 3 (low-level) evidence
[6]
Weston AP, Hiatal hernia with cameron ulcers and erosions. Gastrointestinal endoscopy clinics of North America. 1996 Oct;
[PubMed PMID: 8899401]
[7]
Johnson DA,Ruffin WK, Hiatal hernia. Gastrointestinal endoscopy clinics of North America. 1996 Jul;
[PubMed PMID: 8803572]
[8]
Gray DM,Kushnir V,Kalra G,Rosenstock A,Alsakka MA,Patel A,Sayuk G,Gyawali CP, Cameron lesions in patients with hiatal hernias: prevalence, presentation, and treatment outcome. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus. 2015 Jul;
[PubMed PMID: 24758713]
[9]
Nguyen N,Tam W,Kimber R,Roberts-Thomson IC, Gastrointestinal: Cameron's erosions. Journal of gastroenterology and hepatology. 2002 Mar;
[PubMed PMID: 11982707]
[10]
Fakhre Yaseri H, Gender is a risk factor in patients with gastroesophageal reflux disease. Medical journal of the Islamic Republic of Iran. 2017;
[PubMed PMID: 29445687]
[12]
Katz J,Brar S,Sidhu JS, Histopathological characterization of a Cameron lesion. International journal of surgical pathology. 2012 Oct;
[PubMed PMID: 22614163]
[13]
Zullo A,Manta R,De Francesco V,Fiorini G,Lahner E,Vaira D,Annibale B, Cameron lesions: A still overlooked diagnosis. Case report and systematic review of literature. Clinics and research in hepatology and gastroenterology. 2018 Dec;
[PubMed PMID: 29910147]
Level 3 (low-level) evidence
[14]
Camus M,Jensen DM,Ohning GV,Kovacs TO,Ghassemi KA,Jutabha R,Machicado GA,Dulai GS,Hines OJ, Severe upper gastrointestinal hemorrhage from linear gastric ulcers in large hiatal hernias: a large prospective case series of Cameron ulcers. Endoscopy. 2013;
[PubMed PMID: 23616128]
Level 2 (mid-level) evidence
[15]
Holt JM,Mayet FG,Warner GT,Callender ST,Gunning AJ, Iron absorption and blood loss in patients with hiatus hernia. British medical journal. 1968 Jul 6;
[PubMed PMID: 5301883]
[16]
Zaman A,Katon RM, Push enteroscopy for obscure gastrointestinal bleeding yields a high incidence of proximal lesions within reach of a standard endoscope. Gastrointestinal endoscopy. 1998 May;
[PubMed PMID: 9609429]
[19]
Lin CC,Chen TH,Ho WC,Chen TY, Endoscopic treatment of a Cameron lesion presenting as life-threatening gastrointestinal hemorrhage. Journal of clinical gastroenterology. 2001 Nov-Dec;
[PubMed PMID: 11606864]
[20]
Pauwelyn KA,Verhamme M, Large hiatal hernia and iron deficiency anaemia: clinico-endoscopical findings. Acta clinica Belgica. 2005 Sep-Oct;
[PubMed PMID: 16279396]
[21]
Rothstein R,Filipi C,Caca K,Pruitt R,Mergener K,Torquati A,Haber G,Chen Y,Chang K,Wong D,Deviere J,Pleskow D,Lightdale C,Ades A,Kozarek R,Richards W,Lembo A, Endoscopic full-thickness plication for the treatment of gastroesophageal reflux disease: A randomized, sham-controlled trial. Gastroenterology. 2006 Sep;
[PubMed PMID: 16952539]
Level 1 (high-level) evidence
[23]
Mehershahi S,Jog A,Ronderos DM,Shaikh D,Ihimoyan A, Cameron Ulcers: Rare Case of Overt Upper Gastrointestinal Bleed in a Patient with Alcohol Use Disorder. Cureus. 2020 Apr 12;
[PubMed PMID: 32411546]
Level 3 (low-level) evidence
[24]
Cougard P,Sala JJ,Favre JP,Viard H, [Ulcers of the neck of hiatal hernias. 15 cases]. Journal de chirurgie. 1985 Jun-Jul;
[PubMed PMID: 3876349]
Level 3 (low-level) evidence