Introduction
Shigellosis is an acute diarrheal infection caused by an enteroinvasive gram-negative, facultative anaerobic bacillus of the genus Shigella.[1] Shigellosis is common in developing countries, and transmission is via the fecal-oral route. Infection can occur via person-to-person contact or after ingesting contaminated food or water, especially in poor sanitation conditions. Recent reports reveal increased shigellae transmission via sexual contact, especially between men who have sex with men.[2] Shigellosis can affect all persons across all age groups, but the very young, the elderly, and the immunocompromised are at significantly increased risk.[1]
Shigellae are less susceptible to destruction by gastric acid when passing through the stomach; a small inoculum, as few as 10 to 100 organisms, is required to cause disease. After leaving the stomach, shigellae multiply in the small intestine and enter the colon. In the colon, this species secretes virulence factors that cause extreme inflammation and mediate enterotoxic effects to allow colonization and invasion of the colonic epithelium. Shigellae produce 3 enterotoxins, which cause watery or bloody diarrhea and infection-associated symptoms, such as tenesmus, malaise, and fever.[1]
The average incubation period of shigellae is 1 to 4 days following ingestion of the inoculum. The main symptom of shigellosis is bloody and often mucoid diarrhea; abdominal pain and vomiting are common.[1][3][4][5][6] Shigellosis is typically self-limited and resolves within 5 to 7 days. Antibiotics may be required to shorten the duration of illness or prevent complications, particularly in individuals at increased risk for severe disease and complications, such as hemolytic-uremic syndrome and postreactive arthritis.[7][8] However, antibiotic resistance is rising within the genus, and extensively drug-resistant shigellae have been identified.[2] Antimicrobial susceptibility testing is critical to ensure appropriate therapeutic selection.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiologic agent of shigellosis is a gram-negative, nonmotile, facultatively anaerobic, non–spore-forming bacillus of the genus Shigella and the Enterobacterales order.[1]There are four Shigella species, each representing 1 of the 4 serogroups A to D. Each serogroup comprises 1 or more serotypes and has unique biochemical and virulence characteristics. The shigellae serogroups are:
- Serogroup A: Shigella dysenteriae (12 serotypes)
- Serogroup B: Shigella flexneri (6 serotypes)
- Serogroup C: Shigella boydii (23 serotypes)
- Serogroup D: Shigella sonnei (1 serotype)
S flexneri is the most common cause of diarrhea worldwide and is endemic in low- and middle-income countries.[1] Although S boydii and S dysenteriae are the least common causes of shigellosis, they are endemic in sub-Saharan Africa and South Asia. S dysenteriae type 1 has a high mortality rate and is a frequent cause of shigellosis outbreaks, causing epidemics in populations going through upheaval.[1]. S sonnei is the most common cause of endemic diarrhea in high-income countries and an important cause of travelers' diarrhea. S sonnei usually causes mild disease limited to watery diarrhea, while S flexneri and S dysenteriae usually cause dysentery with bloody diarrhea.[6][9] In the United States, shigellosis is the most common cause of bacterial gastroenteritis, accounting for 500,000 cases, 100,000 hospitalizations, and 500 deaths annually; 77% of cases are caused by S sonnei.[10]
Epidemiology
Humans are the only natural reservoir for shigellae. In high-income countries, the transmission of Shigella is mostly via the fecal-oral route or by direct person-to-person contact, including sexual contact. Transmission frequently occurs between people with poor hygiene and incontinence, such as young children, those in long-term care facilities, or areas of crowding.[11][12] In low- and middle-income countries, transmission via the fecal-oral route is most commonly from contaminated food or water.[13]
The estimated annual global incidence of shigellosis is 188 million cases; approximately 164,000 cases result in death.[14] Shigellosis outbreaks occur whenever war or natural disasters result in unhygienic living conditions, overcrowding, and poor sanitation. Rising temperatures also remarkably increase the risk of diarrheal diseases such as shigellosis.[15]
There is no gender or ethnic predilection for shigellosis. Worldwide, shigellae is the most common cause of dysentery in children aged 6 months to 5 years.[14][16] S flexneri is the serogroup most responsible for shigellosis in children less than 5 years of age in low- and middle-income countries. The second-most common etiologic agent is S sonnei.[17]
Pathophysiology
Shigellosis is characterized by acute inflammatory colitis and bloody diarrhea.[1][18][19] Shigellae is transmitted predominately via fecal-oral or close personal contact.[2] The infecting dose of shigellae is incredibly low; as few as 10 organisms are required to cause disease.[1] Shigellae are acid-resistant and survive passage through the stomach to access the intestine.[1] Shigellae multiply in the small intestine and enter the colon; the colonic mucosa is the site of pathogenic events in shigellosis.
Shigellae cross the colonic mucosal membranes via transcytosis through the basolateral epithelial M cells into macrophages.[20] The bacteria evade the phagolysosome and activate macrophage apoptosis.[21] The release of inflammatory cytokines such as interleukins 1 and 8 activate the innate immune system and initiate an inflammatory response within the colonic mucosa. After release from apoptotic macrophages, shigellae initiate a multistep invasion on the basolateral side of enterocytes that reorganizes the epithelial cytoskeleton, induces actin polymerization, and results in endocytosis of the bacteria into the host cell. Bacterial invasion triggers nuclear factor kappa B, which produces interleukin 8 that calls neutrophils to the invasion site. The resulting inflammatory response further damages the epithelium, degrades the epithelial barrier, and diarrhea ensues.[22][23]
Many shigellae produce up to 3 different enterotoxins, including the enterotoxins designated ShET1 and ShET2 and Shiga toxin (Stx). The chromosomally-encoded ShET1 is most frequently produced by S flexneri, whereas the virulence plasmid-encoded ShET2 can be found in any species. Stx is the main virulence factor produced by S dysenteriae type 1; production of Stx by S sonnei and S flexneri has been reported.[24][25] Stx is not essential for shigellosis symptoms but does contribute to illness severity; the role of Stx in enterocyte injury is uncertain. However, systemic manifestations of Shigella infection, including complications of hemolytic uremic syndrome, are at least partially mediated by Stx.[6]
Enterocyte injury by Shigella-mediated enterotoxins creates ulcers in the intestinal mucosa, particularly the colon, and adds a hemorrhagic component to the pathophysiologic manifestations of shigellosis.[1] Access to the underlying lamina propria further perpetuates the inflammatory response.[8] The resulting small-volume stools containing leukocytes from the inflammatory response, erythrocytes from intestinal ulceration, and bacteria comprise the classic dysentery syndrome of symptomatic shigellosis.[8]
Histopathology
Histopathologic examination of intestinal mucosal biopsies is neither recommended nor required to diagnose shigellosis. However, macroscopic examination of the Shigella-infected tissues reveals inflammation, ulcerative necrosis, hyperemia, and edema. Microscopic examination may reveal epithelial invasion by polymorphonuclear cells and inflammatory patch pseudomembranes.[13]
History and Physical
The clinical manifestations of shigellosis usually begin within 1 to 4 days following exposure. The clinical spectrum of the disease varies with the infecting species and the quality of host defenses. The disease course may be self-limited in immunocompetent persons and last 5 to 7 days. However, the disease is typically more severe and prolonged in young children, older persons, international travelers, individuals living with HIV, and those living in crowded, unsanitary conditions.[26] S sonnei usually causes a mild illness limited to watery diarrhea. S flexneri and S dysenteriae type 1 are more likely to cause bloody diarrhea. When obtaining a medical history from patients with the signs or symptoms of shigellosis, a thorough exposure history, including sexual practices, sick contacts, and recent travel, must be obtained. Approximately 70% to 90% of patients with shigellosis will report abdominal pain ranging from mild discomfort to severe, diffuse, colicky pain. Another 70% to 80% describe small-volume mucoid diarrhea that will precede bloody diarrhea in 30% to 50%.[27] Patients commonly report fever, anorexia, lethargy, fatigue, malaise, and tenesmus.[28]
Patients with an acute diarrheal illness require a complete set of vital signs; fever, tachycardia, tachypnea, and hypotension are suggestive of more severe disease and potential dehydration. The abdominal examination may range from essentially normal to distention, diffuse tenderness, and hyperactive bowel sounds, particularly in the left lower quadrant. Severe disease may result in altered mental status, frank delirium, encephalopathy, seizures, meningismus, and coma. Evidence of acute kidney injury, hemolytic uremic syndrome, toxic megacolon, or reactive arthritis are indicative of severe shigellosis.[29][30]
Evaluation
Stool Testing and Culture
The diagnosis of shigellosis is confirmed when the bacteria is identified in the feculent material of a symptomatic person. A stool sample should be obtained from patients with shigellosis-type symptoms or signs for analysis and culture; stool samples are superior to rectal swabs. Approximately 70% of stool samples from patients with shigellosis will reveal fecal leukocytes and blood.[31] Stool alpha-1 antitrypsin assays, enzyme-linked immunosorbent assays (ELISAs), and polymerase chain reaction (PCR) testing are not required to diagnose shigellosis.[32] However, stool alpha-1 antitrypsin levels can be elevated in the acute phases of shigellosis and will remain high in patients who fail medical therapy. In rare circumstances, culture-independent diagnostic tests like ELISA or PCR may be required to detect bacterial genetic material or toxins in order to make a diagnosis. Culture-independent diagnostic tests also promote the tracking and prevention of outbreaks and the detection of antibiotic resistance patterns.[32]
Blood cultures may be recommended in patients with severe cases of shigellosis.[33] While most patients with shigellosis will not have Shigella-associated bacteremia, bacteremia is associated with increased mortality rates.[34][35] Bacteremia is most likely to occur in patients at increased risk of severe disease, such as extremes of age and those who are immunocompromised.
Laboratory Studies
Laboratory studies are not required to diagnose shigellosis. However, patients with evidence of dehydration, severe disease, or clinical complications will benefit from diagnostic laboratory evaluations. A complete blood count may reveal a leukocytosis with a left shift; anemia and thrombocytopenia may be present. When anemia and thrombocytopenia are accompanied by schistocyte formation, hemolytic uremic syndrome should be suspected.[36][37]
Mild transaminase elevations and electrolyte disturbances may be seen in patients with severe shigellosis. However, hyponatremia is most commonly due to the syndrome of inappropriate antidiuretic hormone secretion.[31][38] Azotemia may be evidence of dehydration or hemolytic uremic syndrome.[31][38] Inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein are frequently elevated in severe disease but are nondiagnostic.
Treatment / Management
The mainstay of shigellosis treatment is supportive care consisting mainly of hydration and electrolyte management. Oral rehydration might be adequate in many cases; in more severe cases, intravenous fluid rehydration with or without hospitalization may be necessary. Antimotility drugs such as loperamide, paregoric, or diphenoxylate are not recommended for patients with Shigella infection; these medications may worsen the condition and cause toxic megacolon.[39] Antimicrobial therapy is not usually recommended for mild cases of shigellosis. Antimicrobials are recommended if a patient requires hospitalization, is bacteremic or septic, is immunocompromised, is a food handler, or works with small children or in institutions. Antimicrobials decrease the symptoms of shigellosis by 2 days and can limit transmission to others.[32] The choice of antimicrobial therapy for shigellosis should be made after accounting for local antimicrobial resistance data and the risk profile of each patient, which includes recent travel history if the infection was acquired in Asia or Africa, their sexual practices, previous antimicrobial use, people experiencing homelessness, and immunosuppression.(A1)
Antimicrobial resistance in Shigella has been increasing worldwide and is a significant public health problem. The resistance mechanisms arise in Shigella by either plasmid-encoded resistance or horizontal transfer of plasmids, which transfer antimicrobial-resistant genes between bacteria. Using whole-genome sequencing, it has been possible to follow the path of these resistant genetic elements worldwide.[40] The emergence of extremely drug-resistant (XDR) S sonnei and S flexneri outbreak strains is caused by the international dissemination of sexually transmissible strains of shigellae, particularly in Great Britain and Europe.[40] These strains carry a plasmid-encoded extended-spectrum β-lactamase (ESBL) gene, blaCTX-M-27, that renders these bacteria penicillin- and cephalosporin-resistant; this plasmid can be transmitted to bacterial genera.[40] Other plasmids, including IncFII, have resulted in resistance to azithromycin.[41] Yet other ESBL genes have expanded the prevalence of ESBL-producing shigellae.[41] Rising antimicrobial resistance is concerning because macrolides, penicillins, cephalosporins, and fluoroquinolones can no longer be employed with any empiric therapeutic certainty in patients with suspected or documented shigellosis. (B2)
There are no steadfast rules for the treatment of shigellosis. In general, if a patient is in the outpatient setting and is at low risk for antimicrobial resistance, fluoroquinolones are the drug of choice for empiric therapy; azithromycin and trimethoprim-sulfamethoxazole are also options in these patients.[42] Third-generation cephalosporins are recommended in high-risk patients, including infected patients in Africa and Asia, international travelers, patients with HIV, and men who have sex with men.[43][12] Carbapenem antimicrobials may be considered for patients at high risk for antimicrobial resistance, those ill for more than 3 to 5 days, those who have failed outpatient antimicrobial therapy, or have been hospitalized. A recent study from the United Kingdom recommended treating hospitalized patients with severe XDR shigellosis with parenteral carbapenems and colistin or oral pivmecillinam and fosfomycin for patients with a prolonged course of illness or as an oral step-down regimen after intravenous therapy.[44](A1)
In the pediatric population, empiric therapy is azithromycin when antimicrobial susceptibility is unavailable. In a randomized control trial, azithromycin was found to show clinical success in 82% of patients and bacteriological success in 94% of patients.[45] Cefixime and ceftibuten can be used as first-line to treat shigellosis in South Asia due to widespread resistance to commonly used antibiotics.[46] The alternative regimen includes pivmecillinam to effectively decrease diarrhea duration and eradicate Shigella in the stool.[47][48] Parenteral antibiotics are indicated in children when shigellosis is suspected or proven when they are septic, bacteremic, febrile with a temperature above 39 °C (102.2 °F), are immunosuppressed, are infected with HIV, or are unable to take oral medications.[34] Ceftriaxone is recommended in this population as a single dose or for 5 days.(A1)
Frequent handwashing with soap and water is recommended, especially after using the bathroom and before food preparation. Food handlers should not engage in food preparation if stool cultures remain positive.
Differential Diagnosis
The differential diagnosis of shigellosis includes infection with bacteria or viruses that cause a similar clinical picture of fever, nausea, vomiting, and abdominal pain:
- Non-typhoidal Salmonella
- Escherichia coli
- Campylobacter spp
- Clostridioides difficile
- Typhoid fever (Salmonella enterica serotype Typhi and, less frequently, Salmonella enterica serotypes and paratyphi A, B, and C)
- Entamoeba histolytica
- Aeromonas spp.
Non-infectious causes that are included in the differential diagnosis of shigellosis that manifest as chronic diarrhea include:
- Inflammatory bowel disease
- VIPoma
- Hyperthyroidism
- Lactose intolerance
- Celiac disease
- Irritable bowel syndrome.[49]
Prognosis
Shigellosis is usually a self-limited disease and resolves within 5 to 7 days. In more severe cases and when patients seek medical care, the diagnosis of shigellosis should be included in the differential diagnosis, and treatment should be initiated promptly, especially if the patient is a returning traveler. If shigellosis is diagnosed and treated on time, the prognosis is good, and patients usually recover without sequelae.
Certain poor prognostic factors may increase morbidity and mortality. These include delays in administering appropriate therapy if the patient is immunocompromised, prolonged disease duration of more than 7 days, extremes of age, and bacteremia. Bacteremia, which carries a 20% mortality rate, is rare and occurs mainly in malnourished children.[50]
Complications of shigellosis include hemolytic uremic syndrome, defined as a triad of thrombocytopenia, hemolytic anemia, and acute kidney injury. Hemolytic uremic syndrome is a microangiopathic hemolytic anemia; its hallmark is schistocytes seen on a peripheral smear. The mortality rate is up to 50%.[51]
Complications
Complications of Shigella infection include intestinal and systemic complications listed below.[6]
Intestinal Complications
- Colonic perforation - occurs with S flexneri and S dysenteriae type 1. It is a rare occurrence and primarily occurs in infants and malnourished patients.[52]
- Intestinal obstruction - usually in severe disease and S dysenteriae type 1.[53]
- Toxic megacolon - usually occurs in S dysenteriae type 1 infection.[31]
- Proctitis or rectal prolapse - caused by invasion of shigellae into colonic mucosa; the complications are most commonly seen in infants and young children.[6]
Systemic Complications
- Bacteremia can occur rarely, mostly in children younger than 5 years.[33][34][35]
- Hemolytic uremic syndrome: although uncommon, it is one of the most frequent causes of acute kidney injury in young children and infants.[51]
- Moderate to severe hypovolemia.
- Hyponatremia can be seen with S dysenteriae type 1 infection.[38]
- Leukemoid reaction: common in children between 2 and 10 years of age.[54]
- Neurologic symptoms: generalized seizures are the most common neurologic complication and are usually associated with a higher mortality rate.[55][56]
- Reactive arthritis: sterile, inflammatory arthritis usually caused by S flexneri infection. Arthritis can occur alone or in conjunction with conjunctivitis and urethritis.[57]
- Vulvovaginitis with or without diarrhea: can occur in young girls and is associated with painless vaginal discharge.[58]
- Keratitis is a rare complication that should be considered in a young child with keratitis and a history of recent diarrheal illness.[59]
- Acute myocarditis: occurs mostly in children with S sonnei infection.[60]
Deterrence and Patient Education
Patients with shigellosis should be educated about appropriate infection control practices to prevent transmission of shigellae to others. Stool precautions and careful handwashing can prevent the dissemination of shigellosis. Primary preventive measures include universal availability of potable water, improved personal and food hygiene, and provision of sanitation methods.[61]
Providers should recommend to their patients to:
- Stay home from school, the healthcare environment, jobs in childcare, and food handling when they are sick or until it is safe to return to work as per the national guidance.
- Avoid any sexual contact (anal, oral, penile, or vaginal) while they are still having diarrhea and for 2 weeks after it ends. Handwashing should be frequent and be performed with soap and water for 20 seconds, particularly after using the toilet and cleaning up after someone who is sick. During this period, they should not prepare food for others. Patients should also abstain from using water playgrounds, hot tubs, oceans, lakes, and rivers.
Pearls and Other Issues
Although no vaccine has been developed to prevent shigellosis, measures can be taken to avoid transmitting shigellae. Regularly implementing these measures, especially in high-risk settings and during high-risk practices, could prevent the disease altogether or minimize the severity of illness in those infected. These infection prevention measures include:
- Perform frequent and thorough handwashing with soap and water, especially before meals and in case of contact with people at high risk of transmitting shigellae.
- Perform supervised handwashing for children in their homes and daycare centers, especially when children are not yet toilet-trained.
- Handle and dispose of diapers cautiously when children are not toilet-trained and have a shigellae infection.
- Drink boiled or treated water and avoid eating raw, poorly handled food from vendors when visiting developing countries.
- Avoid sexual contact with people who have diarrhea, confirmed shigellosis, or have recently recovered from diarrheal illness.
- Practice safe sex.
- Avoid using swimming pools during or recently after a diarrheal illness.
Enhancing Healthcare Team Outcomes
The definitive diagnosis of shigellosis will need time to wait for cultures to be obtained and for results to be available. Shigellosis may mimic many other diarrheal diseases, and patients can have a self-limiting course or may be severely ill. Managing patients with suspected or proven shigellosis by an interprofessional team that includes an emergency department physician, an infectious disease specialist, a gastroenterologist, and an internist is essential to ensure that the management is done appropriately and in a timely fashion. The mainstay of treatment of shigellosis is supportive measures, eg administering fluids and electrolytes.
Even though most infections with shigellae are self-limited and do not require antibiotics, antimicrobial therapy may be indicated in either severe disease or high-risk groups. Antibiotic susceptibility testing is highly recommended, as drug resistance is common and may vary regionally. Immunity can occur after a shigella infection and appears to be serogroup-specific.[62][63][64][65]
Because there is no vaccine to prevent the infection, the primary care provider and nurse specialist are vital in educating the public about prevention. This includes handwashing with soap and water, maintaining good personal hygiene, drinking boiled water while traveling, and avoiding sexual contact with a patient recently diagnosed with shigellosis.[66][67]
The outlook for patients treated promptly is good, but delays in treatment can lead to severe disease and poor outcomes with high mortality, especially in high-risk groups.[68] Confirmed cases of shigellosis in the United States should be reported to the Centers for Disease Control and Prevention via state or local health departments.
Media
(Click Image to Enlarge)

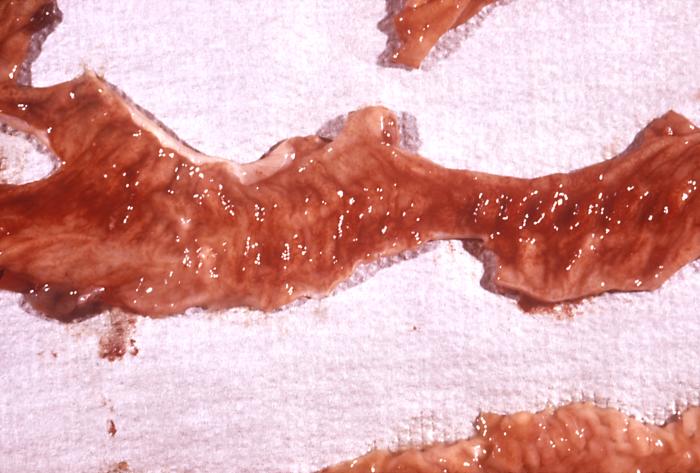
This photomicrograph revealed stool exudates in a patient with shigellosis, which is also known as “Shigella dysentery”, or “Bacterial dysentery”. Usually, those who are infected with Shigella develop diarrhea, which is often bloody, fever, and stomach cramps starting a day or two after they are exposed to the bacterium. Shigellosis usually resolves in 5 to 7 days.
Contributed by The Centers for Disease Control and Prevention (CDC)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
DuPont HL, Levine MM, Hornick RB, Formal SB. Inoculum size in shigellosis and implications for expected mode of transmission. The Journal of infectious diseases. 1989 Jun:159(6):1126-8 [PubMed PMID: 2656880]
Mason LCE, Greig DR, Cowley LA, Partridge SR, Martinez E, Blackwell GA, Chong CE, De Silva PM, Bengtsson RJ, Draper JL, Ginn AN, Sandaradura I, Sim EM, Iredell JR, Sintchenko V, Ingle DJ, Howden BP, Lefèvre S, Njamkepo E, Weill FX, Ceyssens PJ, Jenkins C, Baker KS. The evolution and international spread of extensively drug resistant Shigella sonnei. Nature communications. 2023 Apr 8:14(1):1983. doi: 10.1038/s41467-023-37672-w. Epub 2023 Apr 8 [PubMed PMID: 37031199]
Stoll BJ, Glass RI, Huq MI, Khan MU, Banu H, Holt J. Epidemiologic and clinical features of patients infected with Shigella who attended a diarrheal disease hospital in Bangladesh. The Journal of infectious diseases. 1982 Aug:146(2):177-83 [PubMed PMID: 7108270]
Barrett-Connor E, Connor JD. Extraintestinal manifestations of shigellosis. The American journal of gastroenterology. 1970 Mar:53(3):234-45 [PubMed PMID: 5435635]
Echeverria P, Sethabutr O, Pitarangsi C. Microbiology and diagnosis of infections with Shigella and enteroinvasive Escherichia coli. Reviews of infectious diseases. 1991 Mar-Apr:13 Suppl 4():S220-5 [PubMed PMID: 2047641]
Khan WA, Griffiths JK, Bennish ML. Gastrointestinal and extra-intestinal manifestations of childhood shigellosis in a region where all four species of Shigella are endemic. PloS one. 2013:8(5):e64097. doi: 10.1371/journal.pone.0064097. Epub 2013 May 17 [PubMed PMID: 23691156]
Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, Cantey J, Pickering LK. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Nov 29:65(12):1963-1973. doi: 10.1093/cid/cix959. Epub [PubMed PMID: 29194529]
Level 1 (high-level) evidenceMattock E, Blocker AJ. How Do the Virulence Factors of Shigella Work Together to Cause Disease? Frontiers in cellular and infection microbiology. 2017:7():64. doi: 10.3389/fcimb.2017.00064. Epub 2017 Mar 24 [PubMed PMID: 28393050]
McCrickard LS, Crim SM, Kim S, Bowen A. Disparities in severe shigellosis among adults - Foodborne diseases active surveillance network, 2002-2014. BMC public health. 2018 Feb 7:18(1):221. doi: 10.1186/s12889-018-5115-4. Epub 2018 Feb 7 [PubMed PMID: 29415691]
Hines JZ, Jagger MA, Jeanne TL, West N, Winquist A, Robinson BF, Leman RF, Hedberg K. Heavy precipitation as a risk factor for shigellosis among homeless persons during an outbreak - Oregon, 2015-2016. The Journal of infection. 2018 Mar:76(3):280-285. doi: 10.1016/j.jinf.2017.11.010. Epub 2017 Dec 5 [PubMed PMID: 29217465]
Centers for Disease Control and Prevention (CDC). Shigella flexneri serotype 3 infections among men who have sex with men--Chicago, Illinois, 2003-2004. MMWR. Morbidity and mortality weekly report. 2005 Aug 26:54(33):820-2 [PubMed PMID: 16121121]
Bowen A, Eikmeier D, Talley P, Siston A, Smith S, Hurd J, Smith K, Leano F, Bicknese A, Norton JC, Campbell D, Centers for Disease Control and Prevention (CDC). Notes from the Field: Outbreaks of Shigella sonnei Infection with Decreased Susceptibility to Azithromycin Among Men Who Have Sex with Men - Chicago and Metropolitan Minneapolis-St. Paul, 2014. MMWR. Morbidity and mortality weekly report. 2015 Jun 5:64(21):597-8 [PubMed PMID: 26042652]
Level 3 (low-level) evidenceNiyogi SK. Shigellosis. Journal of microbiology (Seoul, Korea). 2005 Apr:43(2):133-43 [PubMed PMID: 15880088]
Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AKM. Shigellosis. Lancet (London, England). 2018 Feb 24:391(10122):801-812. doi: 10.1016/S0140-6736(17)33296-8. Epub 2017 Dec 16 [PubMed PMID: 29254859]
Liang M, Ding X, Wu Y, Sun Y. Temperature and risk of infectious diarrhea: a systematic review and meta-analysis. Environmental science and pollution research international. 2021 Dec:28(48):68144-68154. doi: 10.1007/s11356-021-15395-z. Epub 2021 Jul 15 [PubMed PMID: 34268683]
Level 1 (high-level) evidenceArnold SLM. Target Product Profile and Development Path for Shigellosis Treatment with Antibacterials. ACS infectious diseases. 2021 May 14:7(5):948-958. doi: 10.1021/acsinfecdis.0c00889. Epub 2021 Mar 10 [PubMed PMID: 33689318]
Shad AA, Shad WA. Shigella sonnei: virulence and antibiotic resistance. Archives of microbiology. 2021 Jan:203(1):45-58. doi: 10.1007/s00203-020-02034-3. Epub 2020 Sep 14 [PubMed PMID: 32929595]
Mellouk N, Enninga J. Cytosolic Access of Intracellular Bacterial Pathogens: The Shigella Paradigm. Frontiers in cellular and infection microbiology. 2016:6():35. doi: 10.3389/fcimb.2016.00035. Epub 2016 Apr 5 [PubMed PMID: 27092296]
Killackey SA, Sorbara MT, Girardin SE. Cellular Aspects of Shigella Pathogenesis: Focus on the Manipulation of Host Cell Processes. Frontiers in cellular and infection microbiology. 2016:6():38. doi: 10.3389/fcimb.2016.00038. Epub 2016 Mar 31 [PubMed PMID: 27066460]
Mathan MM, Mathan VI. Morphology of rectal mucosa of patients with shigellosis. Reviews of infectious diseases. 1991 Mar-Apr:13 Suppl 4():S314-8 [PubMed PMID: 2047656]
Zychlinsky A, Thirumalai K, Arondel J, Cantey JR, Aliprantis AO, Sansonetti PJ. In vivo apoptosis in Shigella flexneri infections. Infection and immunity. 1996 Dec:64(12):5357-65 [PubMed PMID: 8945588]
Level 3 (low-level) evidenceAshida H, Mimuro H, Sasakawa C. Shigella manipulates host immune responses by delivering effector proteins with specific roles. Frontiers in immunology. 2015:6():219. doi: 10.3389/fimmu.2015.00219. Epub 2015 May 7 [PubMed PMID: 25999954]
Gopal A, Chidambaram IS, Devaraj N, Devaraj H. Shigella dysenteriae infection activates proinflammatory response through β-catenin/NF-κB signaling pathway. PloS one. 2017:12(4):e0174943. doi: 10.1371/journal.pone.0174943. Epub 2017 Apr 21 [PubMed PMID: 28430783]
Lamba K, Nelson JA, Kimura AC, Poe A, Collins J, Kao AS, Cruz L, Inami G, Vaishampayan J, Garza A, Chaturvedi V, Vugia DJ. Shiga Toxin 1-Producing Shigella sonnei Infections, California, United States, 2014-2015. Emerging infectious diseases. 2016 Apr:22(4):679-86. doi: 10.3201/eid2204.151825. Epub [PubMed PMID: 26982255]
Gray MD, Lampel KA, Strockbine NA, Fernandez RE, Melton-Celsa AR, Maurelli AT. Clinical isolates of Shiga toxin 1a-producing Shigella flexneri with an epidemiological link to recent travel to Hispañiola. Emerging infectious diseases. 2014 Oct:20(10):1669-77. doi: 10.3201/eid2010.140292. Epub [PubMed PMID: 25271406]
Level 2 (mid-level) evidenceIngle DJ, Easton M, Valcanis M, Seemann T, Kwong JC, Stephens N, Carter GP, Gonçalves da Silva A, Adamopoulos J, Baines SL, Holt KE, Chow EPF, Fairley CK, Chen MY, Kirk MD, Howden BP, Williamson DA. Co-circulation of Multidrug-resistant Shigella Among Men Who Have Sex With Men in Australia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019 Oct 15:69(9):1535-1544. doi: 10.1093/cid/ciz005. Epub [PubMed PMID: 30615105]
Mathan VI, Mathan MM. Intestinal manifestations of invasive diarrheas and their diagnosis. Reviews of infectious diseases. 1991 Mar-Apr:13 Suppl 4():S311-3 [PubMed PMID: 2047655]
Sur D, Ramamurthy T, Deen J, Bhattacharya SK. Shigellosis : challenges & management issues. The Indian journal of medical research. 2004 Nov:120(5):454-62 [PubMed PMID: 15591629]
Erqou S, Teferra E, Mulu A, Kassu A. A case of shigellosis with intractable septic shock and convulsions. Japanese journal of infectious diseases. 2007 Sep:60(5):314-6 [PubMed PMID: 17881877]
Level 3 (low-level) evidenceFerrera PC, Jeanjaquet MS, Mayer DM. Shigella-induced encephalopathy in an adult. The American journal of emergency medicine. 1996 Mar:14(2):173-5 [PubMed PMID: 8924141]
Level 3 (low-level) evidenceBennish ML. Potentially lethal complications of shigellosis. Reviews of infectious diseases. 1991 Mar-Apr:13 Suppl 4():S319-24 [PubMed PMID: 2047657]
Christopher PR, David KV, John SM, Sankarapandian V. Antibiotic therapy for Shigella dysentery. The Cochrane database of systematic reviews. 2010 Aug 4:2010(8):CD006784. doi: 10.1002/14651858.CD006784.pub4. Epub 2010 Aug 4 [PubMed PMID: 20687081]
Level 1 (high-level) evidenceMartin T, Habbick BF, Nyssen J. Shigellosis with bacteremia: a report of two cases and a review of the literature. Pediatric infectious disease. 1983 Jan-Feb:2(1):21-6 [PubMed PMID: 6340078]
Level 3 (low-level) evidenceStruelens MJ, Patte D, Kabir I, Salam A, Nath SK, Butler T. Shigella septicemia: prevalence, presentation, risk factors, and outcome. The Journal of infectious diseases. 1985 Oct:152(4):784-90 [PubMed PMID: 4045231]
Davies NE, Karstaedt AS. Shigella bacteraemia over a decade in Soweto, South Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008 Dec:102(12):1269-73. doi: 10.1016/j.trstmh.2008.04.037. Epub 2008 Jun 11 [PubMed PMID: 18550134]
Level 2 (mid-level) evidenceFried D, Maytal J, Hanukoglu A. The differential leukocyte count in shigellosis. Infection. 1982 Jan:10(1):13-4 [PubMed PMID: 7068229]
Halpern Z, Averbuch M, Dan M, Giladi M, Levo Y. The differential leukocyte count in adults with acute gastroenteritis. Scandinavian journal of infectious diseases. 1992:24(2):205-7 [PubMed PMID: 1641598]
Keusch GT, Bennish ML. Shigellosis: recent progress, persisting problems and research issues. The Pediatric infectious disease journal. 1989 Oct:8(10):713-9 [PubMed PMID: 2682504]
DuPont HL, Hornick RB. Adverse effect of lomotil therapy in shigellosis. JAMA. 1973 Dec 24:226(13):1525-8 [PubMed PMID: 4587313]
Level 1 (high-level) evidenceThorley K, Charles H, Greig DR, Prochazka M, Mason LCE, Baker KS, Godbole G, Sinka K, Jenkins C. Emergence of extensively drug-resistant and multidrug-resistant Shigella flexneri serotype 2a associated with sexual transmission among gay, bisexual, and other men who have sex with men, in England: a descriptive epidemiological study. The Lancet. Infectious diseases. 2023 Jun:23(6):732-739. doi: 10.1016/S1473-3099(22)00807-6. Epub 2023 Jan 30 [PubMed PMID: 36731481]
Level 2 (mid-level) evidenceLefèvre S, Njamkepo E, Feldman S, Ruckly C, Carle I, Lejay-Collin M, Fabre L, Yassine I, Frézal L, Pardos de la Gandara M, Fontanet A, Weill FX. Rapid emergence of extensively drug-resistant Shigella sonnei in France. Nature communications. 2023 Jan 28:14(1):462. doi: 10.1038/s41467-023-36222-8. Epub 2023 Jan 28 [PubMed PMID: 36709320]
Williams PCM, Berkley JA. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatrics and international child health. 2018 Nov:38(sup1):S50-S65. doi: 10.1080/20469047.2017.1409454. Epub [PubMed PMID: 29790845]
Level 1 (high-level) evidenceHeiman KE, Karlsson M, Grass J, Howie B, Kirkcaldy RD, Mahon B, Brooks JT, Bowen A, Centers for Disease Control and Prevention (CDC). Notes from the field: Shigella with decreased susceptibility to azithromycin among men who have sex with men - United States, 2002-2013. MMWR. Morbidity and mortality weekly report. 2014 Feb 14:63(6):132-3 [PubMed PMID: 24522098]
Level 3 (low-level) evidenceCharles H, Prochazka M, Thorley K, Crewdson A, Greig DR, Jenkins C, Painset A, Fifer H, Browning L, Cabrey P, Smith R, Richardson D, Waters L, Sinka K, Godbole G, Outbreak Control Team. Outbreak of sexually transmitted, extensively drug-resistant Shigella sonnei in the UK, 2021-22: a descriptive epidemiological study. The Lancet. Infectious diseases. 2022 Oct:22(10):1503-1510. doi: 10.1016/S1473-3099(22)00370-X. Epub 2022 Jul 6 [PubMed PMID: 35809593]
Level 2 (mid-level) evidenceKhan WA, Seas C, Dhar U, Salam MA, Bennish ML. Treatment of shigellosis: V. Comparison of azithromycin and ciprofloxacin. A double-blind, randomized, controlled trial. Annals of internal medicine. 1997 May 1:126(9):697-703 [PubMed PMID: 9139555]
Level 1 (high-level) evidenceRahman M, Shoma S, Rashid H, El Arifeen S, Baqui AH, Siddique AK, Nair GB, Sack DA. Increasing spectrum in antimicrobial resistance of Shigella isolates in Bangladesh: resistance to azithromycin and ceftriaxone and decreased susceptibility to ciprofloxacin. Journal of health, population, and nutrition. 2007 Jun:25(2):158-67 [PubMed PMID: 17985817]
Salam MA, Dhar U, Khan WA, Bennish ML. Randomised comparison of ciprofloxacin suspension and pivmecillinam for childhood shigellosis. Lancet (London, England). 1998 Aug 15:352(9127):522-7 [PubMed PMID: 9716056]
Level 1 (high-level) evidenceTraa BS, Walker CL, Munos M, Black RE. Antibiotics for the treatment of dysentery in children. International journal of epidemiology. 2010 Apr:39 Suppl 1(Suppl 1):i70-4. doi: 10.1093/ije/dyq024. Epub [PubMed PMID: 20348130]
Level 1 (high-level) evidenceShivashankar R, Lichtenstein GR. Mimics of Inflammatory Bowel Disease. Inflammatory bowel diseases. 2018 Oct 12:24(11):2315-2321. doi: 10.1093/ibd/izy168. Epub [PubMed PMID: 29947781]
Moralez EI, Lofland D. Shigellosis with resultant septic shock and renal failure. Clinical laboratory science : journal of the American Society for Medical Technology. 2011 Summer:24(3):147-52 [PubMed PMID: 21905580]
Level 3 (low-level) evidenceSiegler RL. The hemolytic uremic syndrome. Pediatric clinics of North America. 1995 Dec:42(6):1505-29 [PubMed PMID: 8614598]
Azad MA, Islam M, Butler T. Colonic perforation in Shigella dysenteriae 1 infection. Pediatric infectious disease. 1986 Jan-Feb:5(1):103-4 [PubMed PMID: 3511451]
Level 3 (low-level) evidenceBennish ML, Azad AK, Yousefzadeh D. Intestinal obstruction during shigellosis: incidence, clinical features, risk factors, and outcome. Gastroenterology. 1991 Sep:101(3):626-34 [PubMed PMID: 1860627]
Level 2 (mid-level) evidenceButler T, Islam MR, Bardhan PK. The leukemoid reaction in shigellosis. American journal of diseases of children (1960). 1984 Feb:138(2):162-5 [PubMed PMID: 6695872]
Ashkenazi S, Dinari G, Zevulunov A, Nitzan M. Convulsions in childhood shigellosis. Clinical and laboratory features in 153 children. American journal of diseases of children (1960). 1987 Feb:141(2):208-10 [PubMed PMID: 3544808]
Level 2 (mid-level) evidenceKhan WA, Dhar U, Salam MA, Griffiths JK, Rand W, Bennish ML. Central nervous system manifestations of childhood shigellosis: prevalence, risk factors, and outcome. Pediatrics. 1999 Feb:103(2):E18 [PubMed PMID: 9925864]
Porter CK, Choi D, Riddle MS. Pathogen-specific risk of reactive arthritis from bacterial causes of foodborne illness. The Journal of rheumatology. 2013 May:40(5):712-4. doi: 10.3899/jrheum.121254. Epub 2013 Apr 1 [PubMed PMID: 23547220]
Level 2 (mid-level) evidenceMurphy TV, Nelson JD. Shigella vaginitis: report of 38 patients and review of the literature. Pediatrics. 1979 Apr:63(4):511-6 [PubMed PMID: 375177]
Level 2 (mid-level) evidenceTobias JD, Starke JR, Tosi MF. Shigella keratitis: a report of two cases and a review of the literature. The Pediatric infectious disease journal. 1987 Jan:6(1):79-81 [PubMed PMID: 3547292]
Level 3 (low-level) evidenceRubenstein JS, Noah ZL, Zales VR, Shulman ST. Acute myocarditis associated with Shigella sonnei gastroenteritis. The Journal of pediatrics. 1993 Jan:122(1):82-4 [PubMed PMID: 7678291]
Level 3 (low-level) evidenceMani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine. 2016 Jun 3:34(26):2887-2894. doi: 10.1016/j.vaccine.2016.02.075. Epub 2016 Mar 12 [PubMed PMID: 26979135]
Taylor DN, Bodhidatta L, Brown JE, Echeverria P, Kunanusont C, Naigowit P, Hanchalay S, Chatkaeomorakot A, Lindberg AA. Introduction and spread of multi-resistant Shigella dysenteriae I in Thailand. The American journal of tropical medicine and hygiene. 1989 Jan:40(1):77-85 [PubMed PMID: 2644859]
Level 2 (mid-level) evidenceHuan PT, Taylor R, Lindberg AA, Verma NK. Immunogenicity of the Shigella flexneri serotype Y (SFL 124) vaccine strain expressing cloned glucosyl transferase gene of converting bacteriophage SfX. Microbiology and immunology. 1995:39(7):467-72 [PubMed PMID: 8569531]
Level 3 (low-level) evidenceKeren DF, Kern SE, Bauer DH, Scott PJ, Porter P. Direct demonstration in intestinal secretions of an IgA memory response to orally administered Shigella flexneri antigens. Journal of immunology (Baltimore, Md. : 1950). 1982 Jan:128(1):475-9 [PubMed PMID: 7033378]
Level 3 (low-level) evidenceRasolofo-Razanamparany V, Cassel-Beraud AM, Roux J, Sansonetti PJ, Phalipon A. Predominance of serotype-specific mucosal antibody response in Shigella flexneri-infected humans living in an area of endemicity. Infection and immunity. 2001 Sep:69(9):5230-4 [PubMed PMID: 11500390]
Wilmer A, Romney MG, Gustafson R, Sandhu J, Chu T, Ng C, Hoang L, Champagne S, Hull MW. Shigella flexneri serotype 1 infections in men who have sex with men in Vancouver, Canada. HIV medicine. 2015 Mar:16(3):168-75. doi: 10.1111/hiv.12191. Epub [PubMed PMID: 25656740]
Level 2 (mid-level) evidenceDas JK, Tripathi A, Ali A, Hassan A, Dojosoeandy C, Bhutta ZA. Vaccines for the prevention of diarrhea due to cholera, shigella, ETEC and rotavirus. BMC public health. 2013:13 Suppl 3(Suppl 3):S11. doi: 10.1186/1471-2458-13-S3-S11. Epub 2013 Sep 17 [PubMed PMID: 24564510]
Level 1 (high-level) evidenceBaker KS, Dallman TJ, Field N, Childs T, Mitchell H, Day M, Weill FX, Lefèvre S, Tourdjman M, Hughes G, Jenkins C, Thomson N. Horizontal antimicrobial resistance transfer drives epidemics of multiple Shigella species. Nature communications. 2018 Apr 13:9(1):1462. doi: 10.1038/s41467-018-03949-8. Epub 2018 Apr 13 [PubMed PMID: 29654279]