Introduction
Psoriatic arthritis is a chronic inflammatory arthritis associated with psoriasis found in about 20% of patients with psoriatic arthritis.[1] This condition shares many clinical features with other spondyloarthropathies and rheumatoid arthritis. Psoriatic arthritis is usually seronegative, but some patients may be positive for rheumatoid factor and anti-cyclic citrullinated peptide antibodies. Clinical manifestations of psoriatic arthritis are varied and can change over time, evolving from one particular pattern to another. There is a considerable financial and psychological burden associated with this disease. However, significant progress has recently been made to help better understand the disease pathogenesis, which has translated into new therapies.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology and pathogenesis of psoriatic arthritis are not fully understood; they involve a complex interaction between genetic and environmental factors, resulting in immune-mediated inflammation involving the skin, joints, and other organs. Approximately 33% to 50% of patients with psoriatic arthritis have at least one first-degree relative with psoriatic arthritis or psoriasis.[2] Genes related to psoriatic arthritis encompass those within the HLA region, implicated in antigen presentation and immune recognition. Additionally, non-HLA genes play a role in immune activation and inflammation, influencing intracellular signaling, cytokine expression, and T cell effector function. The genetic associations between psoriatic arthritis and psoriasis are not identical; some genes associated with psoriatic arthritis are not associated with psoriasis (the same is true for psoriasis).[3][4] Also, certain genes are associated with specific phenotypes of psoriatic arthritis.[3][5][6]
Genetic associations in psoriatic arthritis include HLA-B*08:01, HLA-B*27:05, HLA-B*38:01, HLA-B*39:01, HLA-B*44:02HLA-B*57:01, and HLA-C*06:02.[6] HLA-B*08:01 is also associated with peripheral arthritis, joint damage, asymmetric sacroiliitis, and ankylosis. HLA-B*27:05 is associated with axial involvement with symmetric sacroiliitis, enthesitis, and dactylitis. HLA-B38 and HLA-B39 genes are associated with polyarthritis.[5][3] HLA-B*44:02/03 is protective and associated with milder disease [6] (see Table 1. Genetic Associations in Psoriatic Arthritis). The subset of patients with symmetric polyarthritis is associated with HLA-DR4 (this gene is associated with RA).
Non-HLA genes associated with psoriatic arthritis include IL-23R.[4][6] These genes produce proteins involved in immune-mediated inflammation, including TNFAIP3, TNF3IP2, REL, FBX19, and PTPN22 [4][6] (see Table 2. Non-HLA Genes Associated with Psoriatic Arthritis).
Psoriasis is more associated with HLA-B*57:01 and HLA-C*06:02 (the PsO susceptibility region 1 or PSORS1) than psoriatic arthritis.[3][7] IL-12B gene is associated with psoriasis but not psoriatic arthritis.[4] (Several other genes associated with psoriatic arthritis and psoriasis are not mentioned here.)[8]
Axial disease on psoriatic arthritis is more complicated. HLA-B27 gene is present in only 20% of patients with axial psoriatic arthritis compared to 80% to 90% in patients with axial spondyloarthritis, including ankylosing spondylitis (with higher occurrences are seen in White patients.)[9][10] Axial disease in patients with psoriatic arthritis includes patients who are HLA-B27 positive and are clinically similar to ankylosing spondylitis with earlier age of onset, more back pain, and radiographically appearing more like ankylosing spondylitis. Patients with axial psoriatic arthritis who are HLA-B27 negative have different clinical features with similar frequency in women and men, with older age of onset, less back pain, more frequent cervical spine disease, with radiographic features including asymmetric sacroiliitis and non-marginal, bulky syndesmophytes—which are often asymmetric.
Some environmental factors are also suspected but have been difficult to confirm. Epidemiological studies have shown an association between streptococcal infection and recent antibiotic exposure.[11][12][13][14] Skin trauma is known to induce flares of psoriatic skin lesions, known as the Koebner phenomenon. There is evidence that joint trauma may induce a flare of arthritis, referred to as the “internal” or “deep” Koebner phenomenon.[13][14][15] Tobacco is a recognized trigger for rheumatoid arthritis in patients with certain HLA-DR genes and appears to be protective against the development of psoriatic arthritis.[14][16][14]
Table 1. Genetic Associations in Psoriatic Arthritis
| Disease Susceptibility Genes | Disease Susceptibility Hapolotyes | Gene or Haplotype | Disease expression | |
| HLA-B*08:01 | HLA-B *08:01-C*07:01 | HLA-B*08:01 |
Peripheral arthritis, joint damage, ankylosis, asymmetric sacroiliitis |
|
| HLA-B*27:05 | HLA-B*27:-5-C*02:02 | HLA-B*27:05 | Axial involvement, symmetric sacroiliitis, enthesitis, dactylitis | |
| HLA-B*38:01 | HLA-B*27:05-C01:02 | HLA-B*27:05-C*01:02:01 | Bone erosions | |
| HLA-B*39:01 | HLA-B*37:01-C*06:02 | HLA-B*44:02/03 | Milder disease | |
| HLA-B*44:02 (protective) | HLA-B*35:01-C*06:02 | HLA-C*01:02:01 | Bone erosions | |
| HLA-C*06:02 | HLA-B*39:01-C*12:03 | HLA-C*06:02 | Milder disease, a longer delay of arthritis after psoriasis [6] | |
| HLA-B*38:01-C*12:03 |
Table 2. Non-HLA Genes Associated with Psoriatic Arthritis
| Disease Susceptibility Gene | Gene Product |
| IL-23R | IL-23 Receptor; involved in the IL-17 pathway |
| TNFAIP3 | TNF Alpha-Induced Protein 3; involved in TNF-alpha-induced inflammation |
| TRAF3IP2 | TRAF3-Interacting Protein 2; helps to activate NF-DappaB or Jun kinase, which are transcription factors inducing the production of proinflammatory cytokines. |
| REL | REL Proto-Oncogene, NF-kappa B subunit; a component of NF-kappa B |
| FBXL19 | F-Box And Leucine-Rich Repeat Protein 19; regulates ubiquitination and degradation of transmembrane receptor interleukin 1 receptor-like 1 |
| PTPN22 | Protein Tyrosine Phosphatase Non-receptor Type 22; protein tyrosine phosphate is involved in immune regulation [6] |
Epidemiology
The epidemiology of psoriatic arthritis is heterogeneous and varies widely among various population groups. This condition has been estimated to have a prevalence of 0.05% to 0.25% in the general population and around 6% to 41% in patients with psoriasis.[17] This variability of psoriatic arthritis in psoriasis is partially due to underdiagnosis. A meta-analysis showed the prevalence of undiagnosed psoriatic arthritis might be as high as 15.5%.[18] The onset of psoriatic arthritis is usually seen in patients in their 30s and 40s and occurs about equally in men and women.[19]
In most patients, the onset of skin disease precedes arthritis (68%); in about 15% of patients, the arthritic manifestations coincide with the skin disease, and in 17% of patients, arthritis occurs before the skin manifestations—making diagnosis more difficult.[20] This latter group is more common in childhood psoriatic arthritis. When examining the occurrence of psoriatic arthritis over time in a population of patients with psoriasis, the annual incidence of psoriatic arthritis was 1.9 to 2.7% per 100 patients with psoriatic arthritis.[21][2] The prevalence of psoriatic arthritis in patients with psoriasis was 1.7% at 5 years, 3.1% at 10 years, 5.1% at 20 years, and 20.5% at 30 years.[22][23] A severe psoriasis phenotype, scalp, intergluteal and perianal psoriasis, presence of nail pitting, low level of education, and uveitis predict the development of psoriatic arthritis in patients with psoriasis.[22][2]
A literature review of the epidemiology of psoriatic arthritis showed the worldwide prevalence of psoriatic arthritis ranges from 0.1% to 1%.[24][1] There is great variability related to geographic and ethnic variation. The prevalence is higher in Europe (0.19%, with Norway at 0.67% and Sweden at 0.02%) and North America (0.13%) and lower in the Middle East (0.01%), South Asia (0.06%), South East Asia (0.05%), East Asia (0.17%; China 0.002%; Taiwan 0.004%; Japan 0.001%), and South America (0.07%). There is also evidence that the prevalence of psoriatic arthritis has been increasing over time.[1]
The prevalence of psoriatic arthritis in patients with psoriasis is 19.7%, with 21.6% in adults and 3.3% in children.[25] The prevalence of psoriatic arthritis in patients with psoriasis was 23.8% using the CASPAR Criteria. Psoriatic arthritis is more strongly associated with severe psoriasis than mild psoriasis (24.6% vs 15.8%). The proportion of patients with psoriasis with psoriatic arthritis varies depending on the geography and ethnicity of the target population, with 22.7% in Europe, 19.5% in North America, 21.5% in South America, 15.5% in Africa, and 14% in Asia.[25]
Pathophysiology
Various genetic risk factors predispose patients to develop psoriatic arthritis and psoriasis.[3][4][5][8] In these patients, an environmental trigger such as infection or mechanical stress initiates a chronic inflammatory process primarily involving the joints and skin, producing IL-23, a central cytokine in the pathogenesis of psoriatic arthritis and psoriasis.[26][27] Macrophages and dendritic cells produce IL-23. The gastrointestinal tract may be a source of IL-23 due to disturbed barrier function or changes in the microbiota.[26][27][8] Enthesitis, inflammation at the site where ligaments, tendons, and joint capsules attach to the bone, is the prominent pathologic lesion in psoriatic arthritis compared to synovitis in rheumatoid arthritis.[28][26]
Distal interphalangeal joints are frequently involved in psoriatic arthritis but not rheumatoid arthritis because these joints have many entheses but very little synovial tissue. In animal models of spondyloarthropathy, IL-23 stimulates resident T cells, which are CD3+, CD4-, CD8-, IL-23R+, and ROR gamma-t+.[29] This stimulation produces IL-17, IL-22, and TNF-alpha, which promote inflammation, bone loss with erosions, and osteoproliferation.[30]
CD8+ T cells, integral to psoriatic arthritis, are substantiated by their connection with HLA Class I alleles, the oligoclonal expansion of CD8+ T cells, and their association with late-stage HIV infection.[27] Other immune cells involved in the pathogenesis include CD4+ T helper 17 cells that produce IL-17 and IL-22; type 3 innate lymphoid cells that produce IL-17 and IL-22; and gamma-delta T cells that produce IL-17 and TNF-alpha.[27][26] These proinflammatory cytokines recruit neutrophils that enter the synovial fluid, activate synoviocytes, promote angiogenesis locally, and activate osteoclasts, resulting in bone destruction and osteoblasts that promote new bone formation.[26][31][27][32]
Insights into basic pathophysiology have driven the development of therapies, including TNF inhibitors. Initially crafted for rheumatoid arthritis and inflammatory bowel disease, these inhibitors are now standard for managing psoriatic arthritis and psoriasis. While IL-17 inhibitors are FDA-approved for treating psoriatic arthritis and psoriasis, they lack efficacy for rheumatoid arthritis. IL-12/23 inhibitors and IL-23 inhibitors are FDA-approved for treating psoriatic arthritis and psoriasis.
History and Physical
The clinical presentation of psoriatic arthritis is varied. The earliest classification of psoriatic arthritis by Moll and Wright included 5 subtypes:
- Oligoarticular arthritis is asymmetric and involves less than 5 small or large joints
- Polyarticular arthritis is usually symmetric and presents similar to rheumatoid arthritis but may involve the distal interphalangeal joints, rheumatoid factor negative
- Distal arthritis is signified by prominent involvement of the distal interphalangeal joints
- Arthritis mutilans is characterized by severe destructive joint disease with deformities, especially in the hands and feet
- Spondyloarthritis pattern with sacroiliitis and spondylitis (occurring with or without peripheral joint disease)
The asymmetric oligoarticular pattern is the most common form of psoriatic arthritis presentation, accounting for at least 60% of psoriatic arthritis cases. However, this is only true in some patient populations. Over time, the majority of patients will develop polyarticular arthritis. In an analysis of 220 patients with psoriatic arthritis, the patterns of arthritis were as follows: oligoarticular arthritis (14%), polyarthritis (40%), distal arthritis (12%), arthritis mutilans (16%) (see Image. Arthritis Mutilans), sacroiliitis and spondylitis (30%).[20]
The Classification of Psoriatic Arthritis (CASPAR) study identified 63% of patients with polyarticular arthritis.[33] Axial disease is often associated with one of the patterns of peripheral arthritis. According to one prospective cross-sectional study, the frequency of radiological axial involvement in psoriatic arthritis was about 42.9%.[34] The distal pattern is less common, occurring in less than 20% of patients, and may be present with axial disease. Arthritis mutilans prevalence can range from 2% to 21%, reflecting different definitions of this entity adopted from various studies.[35]
The classic definition described arthritis mutilans as the most severe form; its presentation is associated with osteolysis leading to digital telescoping, bone resorption, and sacroiliitis. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis initiative in 2012 was able to achieve a consensus on the features related to arthritis mutilans, which involves consideration of specific features of the disease, including digital telescoping, digital shortening, “pencil-in-cup deformities,” osteolysis, and involvement of distal interphalangeal joints and other small joints of the hands.[36] Features of psoriasis associated with an increased risk of axial disease include active skin lesions with induration, pustular psoriasis, nail involvement, and the Koebner phenomenon.[37]
The discrepancies seen in different studies are explained by the heterogeneous patterns of the disease and the fact that many patients experience a transition from one pattern of arthritis to another over time; this is especially true for patients presenting with asymmetric oligoarthritis who often transition to symmetric polyarthritis. Other factors that may account for variations in patterns of psoriatic arthritis may be related to the fact that most of these studies are from referral centers for psoriatic arthritis and may have an overrepresentation of the more severe patterns of psoriatic arthritis, such as arthritis mutilans and distal arthritis. Axial involvement has generally been thought to occur mainly in patients who are HLA-B27 positive. However, this may be an oversimplification. Ankylosing spondylosis with and without psoriasis has epidemiology similar to typical ankylosing spondylosis with a strong association with HLA-B27. Axial psoriatic arthritis appears to be a distinct entity, and compared to ankylosing spondylosis, the prevalence is fairly equal among women and men, has an older age of onset, has a much lower association with HLA-B27 (20% vs 80%-90%), and is associated with HL-B*08 and HLA-B*38. Clinically, patients with axial psoriatic arthritis have different radiographic findings, more common C-spine involvement, but improvement with TNFi and IL-17i, and possibly IL-23i.[9][10][16]
The clinical features of psoriatic arthritis are described in terms of articular and extra-articular manifestations.
Articular/Periarticular Manifestations of Psoriatic Arthritis
- Peripheral arthritis presents in an oligoarticular vs polyarticular pattern
- The periarticular disease includes enthesitis (inflammation around the insertion of ligaments, tendons, or joint capsules), dactylitis (swelling of the entire digit, finger, or toe, “sausage digit”), and tenosynovitis
- Axial disease involving sacroiliac joints, usually asymmetric
- Spondylitis with discontinuous involvement with bulky non-marginal syndesmophytes
Extra-Articular Manifestations of Psoriatic Arthritis
- Psoriatic skin disease usually presents before the onset of arthritis but can occur simultaneously and even before the onset of joint disease. The severity of skin disease does not correlate well with the severity of the articular disease.[38]
- Nail disease is characterized by onycholysis, pitting, and splinter hemorrhages. The severity of nail disease correlates with skin and joint disease severity.[39] This condition is present in 80% to 90% of patients with psoriatic arthritis and is associated with distal interphalangeal joint involvement.
- Ocular disease in the form of uveitis is unlike that associated with ankylosing spondylosis, as it is often chronic, bilateral, and can involve posterior elements.
Evaluation
There are no laboratory tests specifically for psoriatic arthritis. As in most inflammatory diseases, acute phase reactants such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) may be elevated. However, a normal ESR and CRP should not be used to rule out a diagnosis of psoriatic arthritis, as these levels are increased in only about 40% of patients.[40] Rheumatoid factor and anti-cyclic citrullinated peptide antibodies are classically considered absent in psoriatic arthritis. A negative rheumatoid factor is considered a criterion for diagnosing psoriatic arthritis per the Classification of Psoriatic Arthritis (CASPAR) classification criteria. Results from various studies have shown positive rheumatoid factor in about 2% to 10% of patients diagnosed with psoriatic arthritis, and approximately 5% are positive for anti-cyclic citrullinated peptide antibodies.[41][42] Antinuclear antibodies may also be positive in these patients but usually at low titers. Results from a study by Johnson et al, showed antinuclear antibodies at a titer >1:80 in 14% of patients with psoriatic arthritis.[43]
Radiographic changes show some characteristic patterns in psoriatic arthritis: erosive changes, gross joint destruction, joint space narrowing, and “pencil-in-cup” deformity.[44][45] These findings are driven by bone destruction and pathologic new bone formation, often in the same digit or even the same joint, which is a characteristic feature of psoriatic arthritis (eg, bone destruction with bone production). Despite treatment with disease-modifying anti-rheumatic drugs, psoriatic arthritis results in radiographic damage in about 47% of patients during the first 2 years of the disease.[46] The radiological features of peripheral arthritis in hands and feet include erosive changes, new bone formation, bony ankylosis, and joint osteolysis.[47] Entheseal involvement, including erosions and new bone formation, is characteristic in all spondyloarthropathies.[48]
Axial features, including sacroiliitis and spondylitis, are characterized by the formation of syndesmophytes (ossification of the annulus fibrosis). The features differentiating psoriatic arthritis from ankylosing spondylitis are the asymmetric and often unilateral presentation of sacroiliitis, and syndesmophytes in psoriatic arthritis are non-marginal, bulky, asymmetric, and discontinuous skipping vertebral levels. Plain radiography, CT scan, ultrasound, and MRI are all useful in assessing patients with psoriatic arthritis.[49] Imaging modalities such as musculoskeletal ultrasound (MSUS) and MRI are more sensitive than plain radiography for detecting early joint inflammation, damage, and axial changes, including sacroiliitis.[50][51] However, these imaging modalities are not necessary to properly diagnose psoriatic arthritis. MSUS is used frequently in clinics as it is readily accessible and provides dynamic images. MSUS is especially useful in detecting subclinical synovitis, enthesitis, and dactylitis. MSUS features supportive of enthesitis include entheseal thickening, increased vascularity on power doppler, hypoechogenicity, and enthesophyte formation.[52][53] Similarly, dactylitis features on MSUS include synovial and tenosynovial inflammation.[54]
Classification Criteria
The most accepted classification criteria for psoriatic arthritis is the CASPAR criteria, which have been used since 2006.[33] Other classification criteria that clinicians have used include the original Moll and Wright (1973), Bennet (1979), Vassey and Espinoza (1984), and the modified European Spondyloarthropathy Study Group (1991) criteria.[55]
Moll and Wright Criteria (1973)
- Inflammatory arthritis (peripheral arthritis and sacroiliitis or spondylitis)
- The presence of psoriasis
- The absence of serologic tests for rheumatoid factor [44]
CASPAR Criteria (2006)
Clinical Features/Characteristics/Points
- Skin psoriasis: present - 2; previously present -1; family history, patient not affected - 1
- Nail lesions: onycholysis, pitting, hyperkeratosis - 1
- Dactylitis: present or past, documented by a rheumatologist - 1
- Rheumatoid factor: negative by any method except for latex - 1
- Juxta-articular bone formation: distinct from osteophytes - 1
Per CASPAR criteria, psoriatic arthritis is considered present in patients with inflammatory arthritis who have at least 3 points; this has a specificity of 98.7% and a sensitivity of 91.4%.[33]
Treatment / Management
General Principles
- Guide treatment by disease severity, joint damage degree, extra-articular disease extent, patient preference, and other comorbidities.
- Nonpharmacological therapies, including physical and occupational therapy, exercise programs, and smoking cessation, should be strongly encouraged and incorporated into the treatment plan.[56]
- A treat-to-target approach is the most effective way to control disease activity and minimize joint damage.[57][58] Depending on disease extent, chronicity, and other comorbidities, low remission or low disease activity targets should be employed.
- Due to the heterogeneous presentation of psoriatic arthritis, the type of treatment initiated depends on the domains involved, including peripheral arthritis, enthesitis, dactylitis, axial disease, and skin or nail disease.
- In treatment-naïve patients, nonsteroidal anti-inflammatory drugs are generally useful for treating mild peripheral arthritis symptoms.[59]
- Mild-to-moderate peripheral arthritis may be treated with conventional synthetic DMARDs (disease-modifying antirheumatic drugs) such as methotrexate or occasionally sulfasalazine; the latter is ineffective for skin disease.[60]
- Severe peripheral arthritis usually receives treatment with biologic DMARDs, especially TNF (tumor necrosis factor) inhibitors.
- Axial disease and enthesitis share similar treatment approaches, with a minimal role for conventional synthetic DMARDs. Patients who fail non-steroidal anti-inflammatory drugs should automatically transition to biologic DMARDs.
- A TNF inhibitor is usually recommended over an IL-17 inhibitor, IL-12/23 inhibitor, abatacept, or tofacitinib.
- An IL-17 inhibitor is usually recommended over an IL-12/23 inhibitor, abatacept, or tofacitinib.
- An IL-12/23 inhibitor is usually recommended over abatacept or tofacitinib.
- In patients with severe psoriasis, an IL-12/23 inhibitor or an IL-17 inhibitor may be used instead of a TNF inhibitor.
- Tofacitinib may be used instead of a TNF inhibitor in patients who prefer oral medication and do not have severe psoriasis.
- The American College of Rheumatology (ACR) and National Psoriasis Foundation (NPF) 2018 guidelines recommend a TNF inhibitor over conventional synthetic DMARDs (labeled as OSM, oral small molecules) as a first-line treatment in patients with treatment-naïve psoriatic arthritis. (A1)
A summary of various drugs used in treating psoriatic arthritis appears in a tabular form in Table 1.
ACR/NPF 2018 Guidelines Definition of Active Psoriatic Arthritis
Defined as “disease-causing symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining clinician to be due to psoriatic arthritis” based on ≥1 of the following:
- Swollen joints
- Tender joints
- Dactylitis
- Enthesitis
- Axial disease
- Active skin and/or nail involvement
- Extra-articular inflammatory manifestations such as uveitis or inflammatory bowel disease [61]
ACR/NPF 2018 Guidelines for the Treatment of Psoriatic Arthritis
Initial Treatment
Oral small molecule (OSM): methotrexate (MTX), sulfasalazine (SSZ), leflunomide (LEF), cyclosporine (CSA), apremilast
- Treat with tumor necrosis factor (TNF) inhibitors over OSM: may consider OSM such as MTX in patients with mild psoriatic arthritis and psoriasis, patient's preference, and contraindication to TNF inhibitor.
- Treat with TNF inhibitor over IL-17 inhibitor: may consider IL-17 inhibitor in patients with severe psoriasis or contraindication to TNF inhibitor.
- Treat with TNF inhibitor over IL-12/23 inhibitor: may consider IL-12/23 inhibitor in patients with severe psoriasis of contraindication to TNF inhibitor.
- Treat with OSM over IL-17 inhibitor: may consider IL-17 inhibitor in patients with severe psoriasis and/or psoriatic arthritis.
- Treat with OSM over IL-12/23 inhibitor: may consider IL-12/23 inhibitor in patients with severe psoriasis and/or psoriatic arthritis or concomitant inflammatory bowel disease.
- Treat with MTX over non-steroidal anti-inflammatory drugs: may consider these drugs in patients with mild psoriatic arthritis and psoriasis.
- Treat with IL-17 inhibitor over IL-12/23 inhibitor: may consider IL-12/23 inhibitor in patients with concomitant inflammatory bowel disease.
European League Against Rheumatism Recommendations for the Management of Psoriatic Arthritis With Pharmacological Therapies: 2019 Update
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), Disease-modifying antirheumatic drugs (DMARD), Conventional synthetic DMARD (csDMARD), Biologic DMARD (bDMARD), Monoclonal antibody (mab) Targeted synthetic DMARD (tsDMARD), Janus kinase (JAK), Phosphodiesterase-4 (PDE4)[62]
Overarching principles
- Psoriatic arthritis is a heterogeneous and potentially severe disease that may require multidisciplinary treatment.
- Treating patients with psoriatic arthritis should aim for the best care and must be based on a shared decision between the patient and rheumatologist, considering efficacy, safety, and costs.
- Rheumatologists are the specialists who should primarily care for the musculoskeletal manifestations of patients with psoriatic arthritis; if there is significant skin involvement, collaborate with a dermatologist.
- The primary goal in treating psoriatic arthritis is to maximize health-related quality of life by controlling symptoms, preventing structural damage, and normalizing physical and social function through inflammation control as a crucial component.
- When managing psoriatic arthritis, non-musculoskeletal manifestations (eg, skin, eye, and GI tract) and comorbidities (eg, metabolic syndrome, cardiovascular disease, and depression) should be considered.
Recommendations
- Treatment should aim to reach remission or low disease activity target by regular disease activity assessment and appropriate therapy adjustment.
- NSAIDs may be used to relieve musculoskeletal signs and symptoms.
- Local glucocorticoid injections should be considered adjunctive therapy in psoriatic arthritis; systemic glucocorticoids may be used cautiously at the lowest effective dose.
- A csDMARD should be initiated rapidly in patients with polyarthritis, with MTX preferred in those with relevant skin involvement.
- Consider a csDMARD for patients with monoarthritis or oligoarthritis, particularly with poor prognostic factors such as structural damage, high erythrocyte sedimentation rate or c-reactive protein levels, dactylitis, or nail involvement.
- In patients with psoriatic arthritis who have an inadequate response to at least one csDMARD, therapy with a bDMARD should be commenced; when relevant skin involvement is present, an IL-17 inhibitor or IL-12/23 inhibitor may be preferred.
- In patients with peripheral arthritis and an inadequate response to at least one csDMARD and at least one bDMARD, or when a bDMARD is not appropriate, a JAK inhibitor may be considered.
- In patients with mild disease and an inadequate response to at least one csDMARD, in whom neither a bDMARD nor a JAK inhibitor is appropriate, a PDE4 inhibitor may be considered.
- In patients with unequivocal enthesitis and insufficient response to NSAIDs or local glucocorticoid injections, therapy with a bDMARD should be considered.
- In patients with predominantly axial disease, which is active and has an insufficient response to NSAIDs, therapy with a bDAMRD should be considered (according to current practice, this is a TNF inhibitor); when there is relevant skin involvement, IL-17 inhibitor may be preferred.
- In patients who fail to respond adequately to or are intolerant of abDMARD, switching to another bDMARD or tsDMARD should be considered, including a switch within a class.
- In patients with sustained remission, cautious tapering of DMARDs may be considered.
Differential Diagnosis
Psoriatic arthritis shares some clinical features with other inflammatory arthritis, including rheumatoid arthritis, reactive arthritis, and ankylosing spondylosis. Some cases may be difficult to make a precise diagnosis. Unlike psoriatic arthritis, rheumatoid arthritis tends to be symmetrical and generally spares the distal interphalangeal joints. Ankylosing spondylosis has an earlier onset age than psoriatic arthritis, and sacroiliac involvement is usually symmetric rather than asymmetric.
Treatment Planning
Disease Monitoring
As with any other inflammatory arthritis, patients with psoriatic arthritis require regular disease activity monitoring and appropriate changes to therapy based on the measurement of disease activity. Evaluation of all the domains, including peripheral joints, entheses, digits, axial involvement, and skin and nails, is crucial. The following methods assess disease activity in clinical practice and clinical trials.
Various Parameters Used to Assess Disease Activity in Psoriatic Arthritis
- Tender and swollen joint counts of 68 joints and 66 joints, respectively, in peripheral arthritis
- The Bath Ankylosing Spondylitis Disease Activity Index determines axial disease activity and ankylosing spondylosis
- Health-related Quality of Life (QOL), as measured by indices like PsAQOL
- Functional Assessment of Chronic Illness Therapy-Fatigue Scale
- Composite indices like Disease Activity Index for Psoriatic Arthritis, Minimal Disease Activity, America’s College of Rheumatology criteria ACR 20/50/70, Psoriatic Arthritis Response Criteria, and Composite Psoriatic Disease Activity Index
- The Routine Assessment of Patient Index Data, or RAPID3, is useful for assessing disease activity in rheumatoid arthritis, is simple to administer, and does not require any laboratory indices. This assessment compares favorably to other, more complicated measures of disease activity in psoriatic arthritis and is more practical for routine clinical care.[63] The Disease Activity Score using 28 joints is frequently used to measure disease activity in rheumatoid arthritis. However, this assessment is inadequate for psoriatic arthritis as it focuses on peripheral arthritis.
A treat-to-target approach to attain remission or minimal disease activity is strongly recommended.[58] A patient is considered to have achieved minimal disease activity if they meet 5 of the 7 following criteria.[64]
Minimal Disease Activity
- Tender joint count of </= 1
- Swollen joint count </= 1
- Psoriasis Area and Severity Index of </= 1 or body surface area </= 3
- Patient pain visual analog scale (VAS) score </= 15 mm
- Patient global disease activity VAS score </= 20 mm
- Health Assessment Questionnaire score </= 0.5
- Tender entheseal points </= 1
Specific Agents
csDMARDs
Methotrexate
- For patients with mild peripheral joint and skin disease
- Dose: 7.5 to 25 mg weekly by mouth or subcutaneously, usually given with 1 mg of folic acid daily
- Toxicities include oral ulcers, nausea, cytopenias, increased risk of infections, pulmonary toxicity (pneumonitis), and this drug is teratogenic
- Advise patients to avoid drinking excessive alcohol and be cautious with patients who have chronic kidney disease
- Check complete blood count (CBC) and comprehensive metabolic panel (CMP) every 2 to 4 months
Sulfasalazine
- For patients with mild peripheral joint disease but is not helpful for skin disease
- Dose: 1000 to 1500 mg twice daily
- Toxicities: rash, nausea, diarrhea, and elevated liver enzymes on liver function tests, but rarely leukopenia/neutropenia
- Check CBC and CMP every 2 to 4 months
Leflunomide
- For patients with mild peripheral joint and skin disease
- Dose: 10 to 20 mg every day
- Toxicities: diarrhea, hair loss, skin rash, leukopenia, elevated liver enzymes on liver function tests, and weight loss
- Check CBC and CMP every 2 to 4 months
Cyclosporine
- For patients with active skin disease but not peripheral joint disease
- Dose: 2.5 to 5 mg/kg/day twice a day (but usually 3 mg/kg/day)
- Toxicities: decreased kidney function, hypertension, headache, elevated cholesterol, excessive hair growth, and gum hypertrophy
bDMARDs
TNF inhibitors (TNFi)
- For patients with moderate-severe active psoriatic arthritis with peripheral and axial joint and skin diseases
- All TNFIs are FFA-approved for rheumatoid arthritis and ankylosing spondylitis
- Check tuberculosis test before starting, and check CBC and CMP every 3-4 months
Monoclonal antibody (mab) TNFi
- These are effective for uveitis and inflammatory bowel disease
Etanercept
- A p75 TNF-a receptor; IgG Fc fusion protein
- Dose: 50 mg subcutaneously every week
- FDA-approved for psoriatic arthritis and psoriasis but not recommended for patients with uveitis or inflammatory bowel disease
Infliximab
- Chimeric mab to TNF-alpha
- Dose: 5 mg/kg intravenously every 6 weeks after loading
- FDA-approved for psoriatic arthritis and psoriasis
Adalimumab
- Human mab to TNF-alpha
- Dose: 40 mg subcutaneously every 2 weeks
- FDA-approved for psoriatic arthritis and psoriasis
Golimumab
- Human mab to TNF-alpha
- Dose: 50 mg subcutaneously every month or 2 mg/kg intravenously every 2 months
- FDA-approved for psoriatic arthritis and psoriasis
Certolizumab pegol
- Humanized Fab fragment specific for TNF-a conjugated to 40-kDa PEG
- Dose: 200 mg subcutaneously every 2 weeks after loading
- Toxicities: administration reactions, increased risk of infections including mycobacterial and fungal infections, demyelination, congestive heart failure, drug-induced lupus, and paradoxically psoriasis
- FDA-approved for psoriatic arthritis and effective for treating psoriasis
IL-17 inhibitors (IL-17i)
- For patients with moderate-severe active psoriatic arthritis with peripheral and axial joint and skin disease
- Check TB test before starting, and check CBC and CMP every 3-4 months
- IL-17i is ineffective for uveitis or inflammatory bowel syndrome
Secukinumab
- Human mab to IL-17A
- Dose: 150 to 300 mg subcutaneously every month after loading
- FDA-approved for psoriatic arthritis, psoriasis, and ankylosing spondylitis
- Secukinumab at doses of 300 mg every month was effective in patients with psoriatic arthritis and axial disease in a double-blind, randomized trial [65]
Ixekizumab
- Humanized mab to IL-17A
- Dose: 80 mg subcutaneously every month after loading
- FDA-approved for psoriatic arthritis, psoriasis, and ankylosing spondylitis
Brodaulmab
- Human mab to IL-17R
- Dose: 210 mg SC every 2 weeks after loading
- Toxicities: increased risk of infections, including mycobacterial and fungal infections and candida infections
- FDA approved for psoriasis, but not for psoriatic arthritis
IL-12/23 and IL-23 inhibitors (IL12/23i, IL-23i)
- For patients with moderate-severe active psoriatic arthritis with peripheral joint and skin disease
- Check TB test before starting, and check CBC and CMP every 3-4 months.
- IL-12/23i are ineffective for the axial disease of ankylosing spondylitis or non-radiographic axial psoriatic arthritis but may be effective for axial psoriatic arthritis.
Ustekinumab (IL12/23i)
- Human mab to IL-12/23 p40 subunit
- Dose: 45 mg SC every 3 months after loading (90 mg if weight >100 kg)
- FDA-approved for psoriatic arthritis, psoriasis, and Crohn’s disease
Guselkumab (IL-23i)
- Human mab to IL-23 p19 subunit
- Dose: 100 mg SC every 2 months after loading
- FDA-approved for psoriatic arthritis and psoriasis
Tildrakizumab (IL-23i)
- Human mab to p19 subunit of IL-23
- Dose: 100 mg SC every three months after loading
- Toxicities: an increased risk of infections, including mycobacterial and fungal infections
- FDA-approved for psoriatic arthritis and psoriasis
T cell costimulatory inhibitors
- For patients with mild-moderate psoriatic arthritis with peripheral joint disease
- Check tuberculosis test before starting, and check CBC and CMP every 3-4 months
- Minimally effective for psoriasis
Abatacept CTLA4
- IgG Fc fusion protein
- Dose: 125 mg subcutaneously every week or 500 to 1000 mg every month after loading (depending on weight)
- Toxicities: an increased risk of infections, including mycobacterial and fungal infections.
- FDA-approved for psoriatic arthritis and rheumatoid arthritis
In the randomized, placebo-controlled trials of bDMARs, using csDMARDs such as MTX did not improve outcomes for psoriatic arthritis or psoriasis. Analyses of large cohorts of patients with psoriatic arthritis and csDMARDs such as MTX improved clinical outcomes. Using csDMARDs improved TNFi drug survival [66] and remission rates, especially with MTX.[67] Some retrospective studies suggest that patients with psoriasis treated with bDMARDs have a lower risk of developing psoriatic arthritis.[68][69][70]
tsDMARDs
PDE-4 inhibitors
- For patients with mild psoriatic arthritis with peripheral joint and skin disease [71]
- Also effective for enthesitis and dactylitis [72]
- No laboratory testing is needed to monitor therapy
Apremilast
- Dose: 30 mg twice daily after titration
- Toxicities: gastrointestinal intolerance, nausea, diarrhea, weight loss, and depression
Janus kinase inhibitors
- For moderate-severely active psoriatic arthritis with peripheral and axial disease
- Check tuberculosis test before starting. Check CBC, neutrophil count, and CMP every 3 months. Perform a lipid panel
Tofacitinib
- Dose: 5 mg by mouth 2 times a day or extended release, 11 mg by mouth every day
- FDA-approved for psoriatic arthritis, ankylosing spondylitis, rheumatoid arthritis, and ulcerative colitis
Upadicitinib
- Dose: 15 mg daily
- Toxicities: an increased risk of infections, including herpes zoster, tuberculosis, fungal infections, thromboembolism, neutropenia, elevated liver enzymes, elevated cholesterol, and gastrointestinal perforations. There is evidence of an increased risk of cardiovascular disease and cancer [73]
- FDA-approved for psoriatic arthritis, ankylosing spondylitis, rheumatoid arthritis, and ulcerative colitis [74]
Updates in psoriatic arthritis therapeutics
- Bimekizumab is a dual IL-17A, and IL-17F humanized monoclonal antibody. A recent double-blind placebo-controlled phase 3 published trial (BE-COMPLETE) showed superior improvements in joint and skin disease outcomes at week 16 compared with placebo in patients with psoriatic arthritis and inadequate response or intolerance to TNF alpha inhibitors [75]
- Selective oral TYK2 inhibitors were found to be well-tolerated and more effective compared to the placebo in psoriatic arthritis in a phase 2 trial,[76] and a phase 3 clinical trial is currently underway
Prognosis
Psoriatic arthritis is considered an aggressive disease with the potential for significant morbidity and poor quality of life in patients. Some features are harbingers of a severe disease course and poor prognosis. These include a large number of actively inflamed joints or polyarticular presentation, elevated erythrocyte sedimentation rate, clinical or radiographic damage, loss of function, and diminished quality of life.[77]
Complications
Once considered a mild disease, psoriatic arthritis is now considered a debilitating disease requiring targeted treatment with frequent monitoring and follow-up care. Complete symptomatic relief is achievable, but most patients continue to have persistent inflammatory disease.[78] Patients with uveitis will require evaluation and treatment by an ophthalmologist. Patients with psoriatic arthritis have an increased prevalence of comorbidities, including metabolic syndrome, obesity, diabetes mellitus, hyperlipidemia, hypertension, and cardiovascular disease.[79]
Consultations
Psoriatic arthritis is best managed by rheumatologists but in collaboration with dermatologists if they have significant psoriasis.
Deterrence and Patient Education
Patients should be extensively educated and counseled about the chronic nature of psoriatic arthritis and the importance of non-pharmacological measures, including exercise, smoking cessation, weight loss, and physical and occupational therapy. Patients should be aware of the fluctuating nature of this disease, which requires very close monitoring by the multidisciplinary treatment team. The side effects of immunosuppressive medications require a detailed explanation, and an attempt should also be made to educate the patient’s family.
Enhancing Healthcare Team Outcomes
Patients with psoriatic arthritis have a heterogeneous clinical presentation involving various domains. This condition is managed best by an interprofessional team approach to treating articular disease, skin disease, other manifestations, and medical comorbidities. Patient education is vital to ensure symptoms are under control. The physical therapist should encourage exercises to restore joint function. The pharmacist should educate the patient on different medications, their benefits, and adverse reactions, monitor agent selection and dosing, and check for potential drug-drug interactions. Nurses should inform patients of the importance of abstaining from alcohol and discontinuing tobacco, answer questions, and help monitor treatment progress. The dietitian should encourage a healthy diet and weight. A mental health nurse and psychiatrist should be involved, as many patients develop severe anxiety and depression. Patients should be encouraged to seek stress relief. The social worker should assess the home to ensure it can accommodate the patient’s lifestyle. These disciplines need to chart and share their perspectives with the rest of the team so all healthcare team members operate from the same information base and corrective actions can be taken when necessary.
Patients with psoriatic arthritis are also at increased risk of death compared to the general population from cardiovascular diseases such as coronary artery disease leading to angina and myocardial infarction.[80] Thus, reversing the risk factors for ischemic heart disease is vital. Effective interprofessional coordination and communication among rheumatology, dermatology, primary care, nursing staff, pharmacy, and other ancillary healthcare team members are required to attain the best clinical outcome in patients with psoriatic arthritis.
Media
(Click Image to Enlarge)
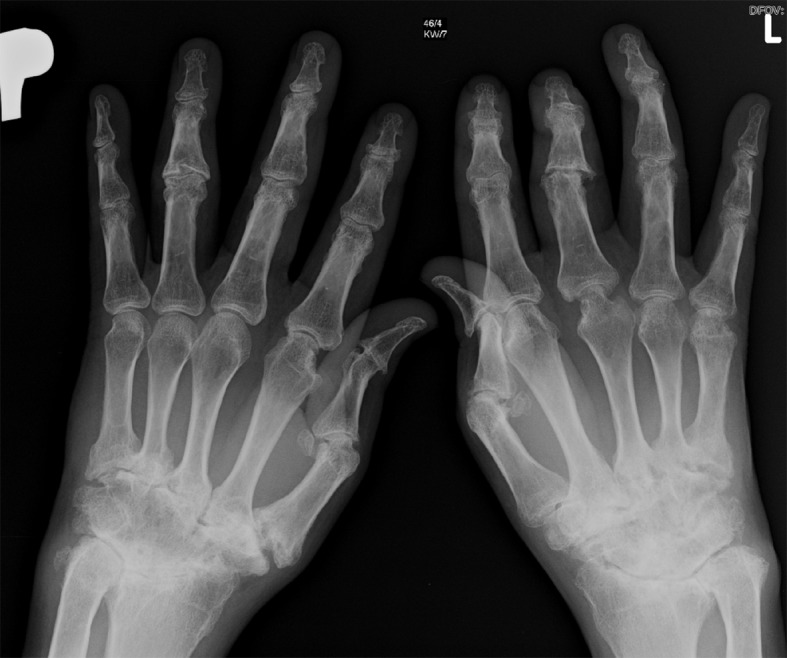
Arthritis Mutilans. X-ray showing the destruction of both hand joints in a patient with arthritis mutilans and psoriatic arthritis. A "pencil in cup" change of the metacarpophalangeal joints is characteristic of this condition.
Sankowski AJ, Lebkowska UM, Cwikła J, Walecka I, Walecki J. Pol J Radiol. 2013;78(1):7-17. doi: 10.12659/PJR.883763.
References
Karmacharya P, Chakradhar R, Ogdie A. The epidemiology of psoriatic arthritis: A literature review. Best practice & research. Clinical rheumatology. 2021 Jun:35(2):101692. doi: 10.1016/j.berh.2021.101692. Epub 2021 May 18 [PubMed PMID: 34016528]
Eder L, Haddad A, Rosen CF, Lee KA, Chandran V, Cook R, Gladman DD. The Incidence and Risk Factors for Psoriatic Arthritis in Patients With Psoriasis: A Prospective Cohort Study. Arthritis & rheumatology (Hoboken, N.J.). 2016 Apr:68(4):915-23. doi: 10.1002/art.39494. Epub [PubMed PMID: 26555117]
FitzGerald O, Haroon M, Giles JT, Winchester R. Concepts of pathogenesis in psoriatic arthritis: genotype determines clinical phenotype. Arthritis research & therapy. 2015 May 7:17(1):115. doi: 10.1186/s13075-015-0640-3. Epub 2015 May 7 [PubMed PMID: 25948071]
Reveille JD. The genetic basis of spondyloarthritis. Annals of the rheumatic diseases. 2011 Mar:70 Suppl 1():i44-50. doi: 10.1136/ard.2010.140574. Epub [PubMed PMID: 21339218]
Gladman DD, Anhorn KA, Schachter RK, Mervart H. HLA antigens in psoriatic arthritis. The Journal of rheumatology. 1986 Jun:13(3):586-92 [PubMed PMID: 3735281]
O'Rielly DD, Rahman P. Clinical and molecular significance of genetic loci associated with psoriatic arthritis. Best practice & research. Clinical rheumatology. 2021 Jun:35(2):101691. doi: 10.1016/j.berh.2021.101691. Epub 2021 May 19 [PubMed PMID: 34020887]
Nair RP, Stuart PE, Nistor I, Hiremagalore R, Chia NVC, Jenisch S, Weichenthal M, Abecasis GR, Lim HW, Christophers E, Voorhees JJ, Elder JT. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. American journal of human genetics. 2006 May:78(5):827-851. doi: 10.1086/503821. Epub 2006 Mar 31 [PubMed PMID: 16642438]
Level 2 (mid-level) evidenceCafaro G, McInnes IB. Psoriatic arthritis: tissue-directed inflammation? Clinical rheumatology. 2018 Apr:37(4):859-868. doi: 10.1007/s10067-018-4012-7. Epub 2018 Feb 23 [PubMed PMID: 29476352]
Feld J, Chandran V, Haroon N, Inman R, Gladman D. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nature reviews. Rheumatology. 2018 Jun:14(6):363-371. doi: 10.1038/s41584-018-0006-8. Epub [PubMed PMID: 29752461]
Feld J, Ye JY, Chandran V, Inman RD, Haroon N, Cook R, Gladman DD. Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? Rheumatology (Oxford, England). 2020 Jun 1:59(6):1340-1346. doi: 10.1093/rheumatology/kez457. Epub [PubMed PMID: 31593590]
Thorleifsdottir RH, Sigurdardottir SL, Sigurgeirsson B, Olafsson JH, Sigurdsson MI, Petersen H, Arnadottir S, Gudjonsson JE, Johnston A, Valdimarsson H. Improvement of psoriasis after tonsillectomy is associated with a decrease in the frequency of circulating T cells that recognize streptococcal determinants and homologous skin determinants. Journal of immunology (Baltimore, Md. : 1950). 2012 May 15:188(10):5160-5. doi: 10.4049/jimmunol.1102834. Epub 2012 Apr 9 [PubMed PMID: 22491250]
Level 1 (high-level) evidenceThrastardottir T, Love TJ. Infections and the risk of psoriatic arthritis among psoriasis patients: a systematic review. Rheumatology international. 2018 Aug:38(8):1385-1397. doi: 10.1007/s00296-017-3873-4. Epub 2017 Nov 9 [PubMed PMID: 29124396]
Level 1 (high-level) evidencePattison E, Harrison BJ, Griffiths CE, Silman AJ, Bruce IN. Environmental risk factors for the development of psoriatic arthritis: results from a case-control study. Annals of the rheumatic diseases. 2008 May:67(5):672-6 [PubMed PMID: 17823200]
Level 2 (mid-level) evidenceEder L, Law T, Chandran V, Shanmugarajah S, Shen H, Rosen CF, Cook RJ, Gladman DD. Association between environmental factors and onset of psoriatic arthritis in patients with psoriasis. Arthritis care & research. 2011 Aug:63(8):1091-7. doi: 10.1002/acr.20496. Epub [PubMed PMID: 21560259]
Level 2 (mid-level) evidenceThorarensen SM, Lu N, Ogdie A, Gelfand JM, Choi HK, Love TJ. Physical trauma recorded in primary care is associated with the onset of psoriatic arthritis among patients with psoriasis. Annals of the rheumatic diseases. 2017 Mar:76(3):521-525. doi: 10.1136/annrheumdis-2016-209334. Epub 2016 Jul 25 [PubMed PMID: 27457510]
Poddubnyy D, Jadon DR, Van den Bosch F, Mease PJ, Gladman DD. Axial involvement in psoriatic arthritis: An update for rheumatologists. Seminars in arthritis and rheumatism. 2021 Aug:51(4):880-887. doi: 10.1016/j.semarthrit.2021.06.006. Epub 2021 Jun 19 [PubMed PMID: 34198146]
Ogdie A, Weiss P. The Epidemiology of Psoriatic Arthritis. Rheumatic diseases clinics of North America. 2015 Nov:41(4):545-68. doi: 10.1016/j.rdc.2015.07.001. Epub 2015 Sep 11 [PubMed PMID: 26476218]
Villani AP, Rouzaud M, Sevrain M, Barnetche T, Paul C, Richard MA, Beylot-Barry M, Misery L, Joly P, Le Maitre M, Aractingi S, Aubin F, Cantagrel A, Ortonne JP, Jullien D. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: Systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2015 Aug:73(2):242-8. doi: 10.1016/j.jaad.2015.05.001. Epub 2015 Jun 6 [PubMed PMID: 26054432]
Level 1 (high-level) evidenceGladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Annals of the rheumatic diseases. 2005 Mar:64 Suppl 2(Suppl 2):ii14-7 [PubMed PMID: 15708927]
Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)--an analysis of 220 patients. The Quarterly journal of medicine. 1987 Feb:62(238):127-41 [PubMed PMID: 3659255]
Eder L, Chandran V, Shen H, Cook RJ, Shanmugarajah S, Rosen CF, Gladman DD. Incidence of arthritis in a prospective cohort of psoriasis patients. Arthritis care & research. 2011 Apr:63(4):619-22. doi: 10.1002/acr.20401. Epub [PubMed PMID: 21452273]
Level 3 (low-level) evidenceWilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis and rheumatism. 2009 Feb 15:61(2):233-9. doi: 10.1002/art.24172. Epub [PubMed PMID: 19177544]
Level 2 (mid-level) evidenceChristophers E, Barker JN, Griffiths CE, Daudén E, Milligan G, Molta C, Sato R, Boggs R. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European dermatology clinics. Journal of the European Academy of Dermatology and Venereology : JEADV. 2010 May:24(5):548-54. doi: 10.1111/j.1468-3083.2009.03463.x. Epub 2009 Oct 23 [PubMed PMID: 19874432]
Level 2 (mid-level) evidenceStolwijk C, van Onna M, Boonen A, van Tubergen A. Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis care & research. 2016 Sep:68(9):1320-31. doi: 10.1002/acr.22831. Epub 2016 Jul 27 [PubMed PMID: 26713432]
Level 1 (high-level) evidenceAlinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, Gottlieb AB, Gisondi P, Wu JJ, Thyssen JP, Egeberg A. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. Journal of the American Academy of Dermatology. 2019 Jan:80(1):251-265.e19. doi: 10.1016/j.jaad.2018.06.027. Epub 2018 Jun 19 [PubMed PMID: 29928910]
Level 1 (high-level) evidenceSchett G, Lories RJ, D'Agostino MA, Elewaut D, Kirkham B, Soriano ER, McGonagle D. Enthesitis: from pathophysiology to treatment. Nature reviews. Rheumatology. 2017 Nov 21:13(12):731-741. doi: 10.1038/nrrheum.2017.188. Epub [PubMed PMID: 29158573]
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. The New England journal of medicine. 2017 May 25:376(21):2095-6. doi: 10.1056/NEJMc1704342. Epub [PubMed PMID: 28538114]
McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet (London, England). 1998 Oct 3:352(9134):1137-40 [PubMed PMID: 9798608]
Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JH, Eberl G, Buckley CD, Kastelein RA, Pierce RH, Laface DM, Cua DJ. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nature medicine. 2012 Jul 1:18(7):1069-76. doi: 10.1038/nm.2817. Epub 2012 Jul 1 [PubMed PMID: 22772566]
Level 3 (low-level) evidenceLories RJ, McInnes IB. Primed for inflammation: enthesis-resident T cells. Nature medicine. 2012 Jul 6:18(7):1018-9. doi: 10.1038/nm.2854. Epub 2012 Jul 6 [PubMed PMID: 22772553]
Level 3 (low-level) evidenceVeale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet (London, England). 2018 Jun 2:391(10136):2273-2284. doi: 10.1016/S0140-6736(18)30830-4. Epub 2018 Jun 1 [PubMed PMID: 29893226]
Stober C. Pathogenesis of psoriatic arthritis. Best practice & research. Clinical rheumatology. 2021 Jun:35(2):101694. doi: 10.1016/j.berh.2021.101694. Epub 2021 Jun 6 [PubMed PMID: 34108102]
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis and rheumatism. 2006 Aug:54(8):2665-73 [PubMed PMID: 16871531]
Jadon DR, Sengupta R, Nightingale A, Lindsay M, Korendowych E, Robinson G, Jobling A, Shaddick G, Bi J, Winchester R, Giles JT, McHugh NJ. Axial Disease in Psoriatic Arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Annals of the rheumatic diseases. 2017 Apr:76(4):701-707. doi: 10.1136/annrheumdis-2016-209853. Epub 2016 Dec 2 [PubMed PMID: 27913376]
Haddad A, Chandran V. Arthritis mutilans. Current rheumatology reports. 2013 Apr:15(4):321. doi: 10.1007/s11926-013-0321-7. Epub [PubMed PMID: 23430715]
Chandran V, Gladman DD, Helliwell PS, Gudbjörnsson B. Arthritis mutilans: a report from the GRAPPA 2012 annual meeting. The Journal of rheumatology. 2013 Aug:40(8):1419-22. doi: 10.3899/jrheum.130453. Epub [PubMed PMID: 23908536]
Belman S, Walsh JA, Carroll C, Milliken M, Haaland B, Duffin KC, Krueger GG, Feng BJ. Psoriasis Characteristics for the Early Detection of Psoriatic Arthritis. The Journal of rheumatology. 2021 Oct:48(10):1559-1565. doi: 10.3899/jrheum.201123. Epub 2021 Apr 15 [PubMed PMID: 33858978]
Cohen MR, Reda DJ, Clegg DO. Baseline relationships between psoriasis and psoriatic arthritis: analysis of 221 patients with active psoriatic arthritis. Department of Veterans Affairs Cooperative Study Group on Seronegative Spondyloarthropathies. The Journal of rheumatology. 1999 Aug:26(8):1752-6 [PubMed PMID: 10451073]
Williamson L, Dalbeth N, Dockerty JL, Gee BC, Weatherall R, Wordsworth BP. Extended report: nail disease in psoriatic arthritis--clinically important, potentially treatable and often overlooked. Rheumatology (Oxford, England). 2004 Jun:43(6):790-4 [PubMed PMID: 15113998]
Punzi L, Podswiadek M, Oliviero F, Lonigro A, Modesti V, Ramonda R, Todesco S. Laboratory findings in psoriatic arthritis. Reumatismo. 2007:59 Suppl 1():52-5 [PubMed PMID: 17828345]
Bogliolo L, Alpini C, Caporali R, Scirè CA, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. The Journal of rheumatology. 2005 Mar:32(3):511-5 [PubMed PMID: 15742445]
Level 2 (mid-level) evidenceSilvy F, Bertin D, Bardin N, Auger I, Guzian MC, Mattei JP, Guis S, Roudier J, Balandraud N. Antinuclear Antibodies in Patients with Psoriatic Arthritis Treated or Not with Biologics. PloS one. 2015:10(7):e0134218. doi: 10.1371/journal.pone.0134218. Epub 2015 Jul 31 [PubMed PMID: 26230924]
Johnson SR, Schentag CT, Gladman DD. Autoantibodies in biological agent naive patients with psoriatic arthritis. Annals of the rheumatic diseases. 2005 May:64(5):770-2 [PubMed PMID: 15834057]
Moll JM, Wright V. Psoriatic arthritis. Seminars in arthritis and rheumatism. 1973:3(1):55-78 [PubMed PMID: 4581554]
Siannis F, Farewell VT, Cook RJ, Schentag CT, Gladman DD. Clinical and radiological damage in psoriatic arthritis. Annals of the rheumatic diseases. 2006 Apr:65(4):478-81 [PubMed PMID: 16126794]
Level 2 (mid-level) evidenceKane D, Stafford L, Bresnihan B, FitzGerald O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford, England). 2003 Dec:42(12):1460-8 [PubMed PMID: 14523223]
Sudoł-Szopińska I, Matuszewska G, Kwiatkowska B, Pracoń G. Diagnostic imaging of psoriatic arthritis. Part I: etiopathogenesis, classifications and radiographic features. Journal of ultrasonography. 2016 Mar:16(64):65-77. doi: 10.15557/JoU.2016.0007. Epub 2016 Mar 29 [PubMed PMID: 27104004]
Tan AL, Grainger AJ, Tanner SF, Emery P, McGonagle D. A high-resolution magnetic resonance imaging study of distal interphalangeal joint arthropathy in psoriatic arthritis and osteoarthritis: are they the same? Arthritis and rheumatism. 2006 Apr:54(4):1328-33 [PubMed PMID: 16575858]
Poggenborg RP, Østergaard M, Terslev L. Imaging in Psoriatic Arthritis. Rheumatic diseases clinics of North America. 2015 Nov:41(4):593-613. doi: 10.1016/j.rdc.2015.07.007. Epub 2015 Aug 25 [PubMed PMID: 26476221]
Offidani A, Cellini A, Valeri G, Giovagnoni A. Subclinical joint involvement in psoriasis: magnetic resonance imaging and X-ray findings. Acta dermato-venereologica. 1998 Nov:78(6):463-5 [PubMed PMID: 9833050]
Level 2 (mid-level) evidenceQueiro R, Tejón P, Alonso S, Alperi M, Ballina J. Erosive discovertebral lesion (Andersson lesion) as the first sign of disease in axial psoriatic arthritis. Scandinavian journal of rheumatology. 2013:42(3):220-5. doi: 10.3109/03009742.2012.739637. Epub 2013 Jan 14 [PubMed PMID: 23311864]
Level 3 (low-level) evidenceBalint PV, Sturrock RD. Inflamed retrocalcaneal bursa and Achilles tendonitis in psoriatic arthritis demonstrated by ultrasonography. Annals of the rheumatic diseases. 2000 Dec:59(12):931-3 [PubMed PMID: 11087694]
Level 3 (low-level) evidenceBandinelli F, Prignano F, Bonciani D, Bartoli F, Collaku L, Candelieri A, Lotti T, Matucci-Cerinic M. Ultrasound detects occult entheseal involvement in early psoriatic arthritis independently of clinical features and psoriasis severity. Clinical and experimental rheumatology. 2013 Mar-Apr:31(2):219-24 [PubMed PMID: 23190740]
Level 2 (mid-level) evidenceKane D, Greaney T, Bresnihan B, Gibney R, FitzGerald O. Ultrasonography in the diagnosis and management of psoriatic dactylitis. The Journal of rheumatology. 1999 Aug:26(8):1746-51 [PubMed PMID: 10451072]
Helliwell PS, Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Annals of the rheumatic diseases. 2005 Mar:64 Suppl 2(Suppl 2):ii3-8 [PubMed PMID: 15708931]
Di Minno MN, Peluso R, Iervolino S, Russolillo A, Lupoli R, Scarpa R, CaRRDs Study Group. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor α blockers. Annals of the rheumatic diseases. 2014 Jun:73(6):1157-62. doi: 10.1136/annrheumdis-2012-202812. Epub 2013 Jun 14 [PubMed PMID: 23771989]
Level 1 (high-level) evidenceCoates LC. Treating to target in psoriatic arthritis. Current opinion in rheumatology. 2015 Mar:27(2):107-10. doi: 10.1097/BOR.0000000000000140. Epub [PubMed PMID: 25603035]
Level 3 (low-level) evidenceCoates LC, Moverley AR, McParland L, Brown S, Navarro-Coy N, O'Dwyer JL, Meads DM, Emery P, Conaghan PG, Helliwell PS. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet (London, England). 2015 Dec 19:386(10012):2489-98. doi: 10.1016/S0140-6736(15)00347-5. Epub 2015 Oct 1 [PubMed PMID: 26433318]
Level 1 (high-level) evidenceCuéllar ML, Citera G, Espinoza LR. Treatment of psoriatic arthritis. Bailliere's clinical rheumatology. 1994 May:8(2):483-98 [PubMed PMID: 8076399]
Lie E, van der Heijde D, Uhlig T, Heiberg MS, Koldingsnes W, Rødevand E, Kaufmann C, Mikkelsen K, Kvien TK. Effectiveness and retention rates of methotrexate in psoriatic arthritis in comparison with methotrexate-treated patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2010 Apr:69(4):671-6. doi: 10.1136/ard.2009.113308. Epub 2009 Sep 9 [PubMed PMID: 19740904]
Level 2 (mid-level) evidenceSingh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, Dubreuil M, Dunham J, Husni ME, Kenny S, Kwan-Morley J, Lin J, Marchetta P, Mease PJ, Merola JF, Miner J, Ritchlin CT, Siaton B, Smith BJ, Van Voorhees AS, Jonsson AH, Shah AA, Sullivan N, Turgunbaev M, Coates LC, Gottlieb A, Magrey M, Nowell WB, Orbai AM, Reddy SM, Scher JU, Siegel E, Siegel M, Walsh JA, Turner AS, Reston J. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis care & research. 2019 Jan:71(1):2-29. doi: 10.1002/acr.23789. Epub 2018 Nov 30 [PubMed PMID: 30499259]
Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, Primdahl J, McGonagle DG, Aletaha D, Balanescu A, Balint PV, Bertheussen H, Boehncke WH, Burmester GR, Canete JD, Damjanov NS, Kragstrup TW, Kvien TK, Landewé RBM, Lories RJU, Marzo-Ortega H, Poddubnyy D, Rodrigues Manica SA, Schett G, Veale DJ, Van den Bosch FE, van der Heijde D, Smolen JS. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Annals of the rheumatic diseases. 2020 Jun:79(6):700-712. doi: 10.1136/annrheumdis-2020-217159. Epub [PubMed PMID: 32434812]
Coates LC, Tillett W, Shaddick G, Pincus T, Kavanaugh A, Helliwell PS. Value of the Routine Assessment of Patient Index Data 3 in Patients With Psoriatic Arthritis: Results From a Tight-Control Clinical Trial and an Observational Cohort. Arthritis care & research. 2018 Aug:70(8):1198-1205. doi: 10.1002/acr.23460. Epub 2018 Jun 28 [PubMed PMID: 29112801]
Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Annals of the rheumatic diseases. 2010 Jan:69(1):48-53. doi: 10.1136/ard.2008.102053. Epub [PubMed PMID: 19147615]
Baraliakos X, Gossec L, Pournara E, Jeka S, Mera-Varela A, D'Angelo S, Schulz B, Rissler M, Nagar K, Perella C, Coates LC. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Annals of the rheumatic diseases. 2021 May:80(5):582-590. doi: 10.1136/annrheumdis-2020-218808. Epub 2020 Dec 17 [PubMed PMID: 33334727]
Level 1 (high-level) evidenceThomas ML, Shaddick G, Charlton R, Cavill C, Holland R, Iannone F, Lapadula G, Lopriore S, Závada J, Uher M, Pavelka K, Szczuková L, Sidiropoulos P, Flouri I, Drosos A, Möller B, Nissen MJ, Müller RB, Scherer A, McHugh N, Nightingale A. Tumor Necrosis Factor Inhibitor Monotherapy Versus Combination Therapy for the Treatment of Psoriatic Arthritis: Combined Analysis of European Biologics Databases. The Journal of rheumatology. 2021 Jan 1:48(1):48-57. doi: 10.3899/jrheum.190815. Epub 2020 Apr 1 [PubMed PMID: 32238520]
Lindström U, Di Giuseppe D, Delcoigne B, Glintborg B, Möller B, Ciurea A, Pombo-Suarez M, Sanchez-Piedra C, Eklund K, Relas H, Gudbjornsson B, Love TJ, Jones GT, Codreanu C, Ionescu R, Nekvindova L, Závada J, Atas N, Yolbas S, Fagerli KM, Michelsen B, Rotar Ž, Tomšič M, Iannone F, Santos MJ, Avila-Ribeiro P, Ørnbjerg LM, Østergaard M, Jacobsson LT, Askling J, Nissen MJ. Effectiveness and treatment retention of TNF inhibitors when used as monotherapy versus comedication with csDMARDs in 15 332 patients with psoriatic arthritis. Data from the EuroSpA collaboration. Annals of the rheumatic diseases. 2021 Nov:80(11):1410-1418. doi: 10.1136/annrheumdis-2021-220097. Epub 2021 Jun 3 [PubMed PMID: 34083206]
Acosta Felquer ML, LoGiudice L, Galimberti ML, Rosa J, Mazzuoccolo L, Soriano ER. Treating the skin with biologics in patients with psoriasis decreases the incidence of psoriatic arthritis. Annals of the rheumatic diseases. 2022 Jan:81(1):74-79. doi: 10.1136/annrheumdis-2021-220865. Epub 2021 Jul 19 [PubMed PMID: 34281904]
Gisondi P, Bellinato F, Targher G, Idolazzi L, Girolomoni G. Biological disease-modifying antirheumatic drugs may mitigate the risk of psoriatic arthritis in patients with chronic plaque psoriasis. Annals of the rheumatic diseases. 2022 Jan:81(1):68-73. doi: 10.1136/annrheumdis-2021-219961. Epub 2021 Jun 18 [PubMed PMID: 34144965]
Shalev Rosenthal Y, Schwartz N, Sagy I, Pavlovsky L. Reply. Arthritis & rheumatology (Hoboken, N.J.). 2022 Aug:74(8):1451-1452. doi: 10.1002/art.42123. Epub 2022 Jun 6 [PubMed PMID: 35315255]
Edwards CJ, Blanco FJ, Crowley J, Birbara CA, Jaworski J, Aelion J, Stevens RM, Vessey A, Zhan X, Bird P. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Annals of the rheumatic diseases. 2016 Jun:75(6):1065-73. doi: 10.1136/annrheumdis-2015-207963. Epub 2016 Jan 20 [PubMed PMID: 26792812]
Level 1 (high-level) evidenceKavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, Lespessailles E, Hall S, Hochfeld M, Hu C, Hough D, Stevens RM, Schett G. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Annals of the rheumatic diseases. 2014 Jun:73(6):1020-6. doi: 10.1136/annrheumdis-2013-205056. Epub 2014 Mar 4 [PubMed PMID: 24595547]
Level 1 (high-level) evidenceYtterberg SR, Bhatt DL, Connell CA. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. Reply. The New England journal of medicine. 2022 May 5:386(18):1768. doi: 10.1056/NEJMc2202778. Epub [PubMed PMID: 35507493]
FitzGerald O, Ogdie A, Chandran V, Coates LC, Kavanaugh A, Tillett W, Leung YY, deWit M, Scher JU, Mease PJ. Psoriatic arthritis. Nature reviews. Disease primers. 2021 Aug 12:7(1):59. doi: 10.1038/s41572-021-00293-y. Epub 2021 Aug 12 [PubMed PMID: 34385474]
Merola JF, Landewé R, McInnes IB, Mease PJ, Ritchlin CT, Tanaka Y, Asahina A, Behrens F, Gladman DD, Gossec L, Gottlieb AB, Thaçi D, Warren RB, Ink B, Assudani D, Bajracharya R, Shende V, Coarse J, Coates LC. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet (London, England). 2023 Jan 7:401(10370):38-48. doi: 10.1016/S0140-6736(22)02303-0. Epub 2022 Dec 6 [PubMed PMID: 36495881]
Level 1 (high-level) evidenceMease PJ, Deodhar AA, van der Heijde D, Behrens F, Kivitz AJ, Neal J, Kim J, Singhal S, Nowak M, Banerjee S. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Annals of the rheumatic diseases. 2022 Jun:81(6):815-822. doi: 10.1136/annrheumdis-2021-221664. Epub 2022 Mar 3 [PubMed PMID: 35241426]
Ritchlin CT, Kavanaugh A, Gladman DD, Mease PJ, Helliwell P, Boehncke WH, de Vlam K, Fiorentino D, Fitzgerald O, Gottlieb AB, McHugh NJ, Nash P, Qureshi AA, Soriano ER, Taylor WJ, Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Treatment recommendations for psoriatic arthritis. Annals of the rheumatic diseases. 2009 Sep:68(9):1387-94. doi: 10.1136/ard.2008.094946. Epub 2008 Oct 24 [PubMed PMID: 18952643]
Level 1 (high-level) evidenceGladman DD, Hing EN, Schentag CT, Cook RJ. Remission in psoriatic arthritis. The Journal of rheumatology. 2001 May:28(5):1045-8 [PubMed PMID: 11361187]
Gupta S, Syrimi Z, Hughes DM, Zhao SS. Comorbidities in psoriatic arthritis: a systematic review and meta-analysis. Rheumatology international. 2021 Feb:41(2):275-284. doi: 10.1007/s00296-020-04775-2. Epub 2021 Jan 9 [PubMed PMID: 33423070]
Level 1 (high-level) evidenceGladman DD, Ang M, Su L, Tom BD, Schentag CT, Farewell VT. Cardiovascular morbidity in psoriatic arthritis. Annals of the rheumatic diseases. 2009 Jul:68(7):1131-5. doi: 10.1136/ard.2008.094839. Epub 2008 Aug 12 [PubMed PMID: 18697777]
