Introduction
Aspiration pneumonia is an infectious pulmonary condition triggered by entering bacteria-rich oropharyngeal fluids into the lower respiratory tract. The aspirated fluid may contain oropharyngeal secretions, particulate matter, or gastric content. Aspiration pneumonia manifests across various clinical contexts and primarily affects older adults. It poses significant risks of morbidity and mortality among individuals with learning disabilities and those with gastrointestinal (GI) or neurologic disorders, predisposing them to abnormal swallowing.[1]
Aspiration pneumonia encompasses both community-acquired and healthcare-associated forms, constituting a physiopathological occurrence that lacks a precise definition. According to a recent review following clinical practice guidelines from Japan, aspiration pneumonia diagnosis hinges on the presence of lung inflammation in patients exhibiting evident aspiration, with documented dysphagia, or a clinical condition strongly associated with aspiration or dysphagia.[2] An infectious process, aspiration pneumonia entails the proliferation and invasion of pathogenic bacteria from the inhaled fluid into the pulmonary parenchyma.[3]
Aspiration pneumonitis differs from aspiration pneumonia in clinical presentation. While both occur following an aspiration event, aspiration pneumonitis manifests as a noninfectious chemical lung injury from inhaling sterile fluid or gastric contents.[3]
Clinicians and healthcare institutions must maintain a high suspicion of aspiration pneumonia in patients susceptible to this condition to ensure accurate diagnosis and mitigate complications. However, the existing literature lacks comprehensive guidance in this regard. Moreover, definitively diagnosing aspiration pneumonia, particularly in cases involving multiple microaspirations, poses a significant challenge. Empiric therapy for patients with aspiration pneumonia has also changed, underscoring the necessity for educating clinical providers on typical presentations, diagnostic protocols, and treatment options for aspiration pneumonia.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Natural physiologic defense mechanisms such as the glottis closure and the cough reflex usually prevent aspiration pneumonia. Nevertheless, their failure increases the risk of aspiration and aspiration pneumonia. Various factors contribute to this risk, including altered mental status, neurological disorders, esophageal motility issues, persistent vomiting, and gastric outlet obstruction. Conditions that increase the risk of aspiration in adults include the following:[1][2][3]
- Advanced age
- Cerebrovascular disease, known as post-stroke pneumonia
- Drug overdose
- Alcohol use disorder
- Seizures
- Sedative medication use
- Central nervous system disorders
- Head trauma
- Intracranial masses
- Dementia
- Amyotrophic lateral sclerosis
- Multiple sclerosis
- Parkinson disease
- Pseudobulbar palsy
- Poor mobility and debility (eg, bedridden status)
- Esophageal strictures, motility disorders, and cancers
- Gastroesophageal reflux disease
- Tracheostomy
- Nasogastric tube placement
- Muscular diseases
- Inflammatory myopathies
- Bulbospinal muscular atrophy
- Oculopharyngeal muscular dystrophy
Advanced age correlates strongly with aspiration pneumonia, as many older adults experience silent microaspirations that may not be clinically apparent.[1] Among patients aged 70 or older hospitalized with pneumonia, dysphagia prevalence was reported at 91.7%, with silent aspirations occurring in over 50% of cases. However, advanced age does not directly predict the risk of aspiration pneumonia. Frailty, poor nutritional status, and limited mobility are considered more reliable indicators of aspiration risk in older patients.[3]
According to various estimates, the prevalence of aspiration pneumonia in specific patient populations is as follows:
- Acute stroke patients: between 3% and 50% [1]
- Post-cerebrovascular event silent aspiration: Occurs in 40% to 70% of cases [3]
- Hospitalized patients with Parkinson disease or dementia: up to 11% over 3 months [1]
- Aspiration pneumonia is a common complication of multiple sclerosis, motor neuron diseases, Huntington disease, Down syndrome, and cerebral palsy.[1]
- Head and neck cancer and its treatment increase the aspiration pneumonia risk, with up to 70% of patients developing aspiration pneumonia during their lifetime. This risk in survivors further increases with time.[1]
In addition to the above predisposing factors, a crucial risk factor for aspiration pneumonia is the degree of bacterial colonization in oral secretions. Even minimal aspiration can lead to infection if there's a high density of bacterial colonization in oropharyngeal secretions, providing ample "bacterial load" for inoculation. A population-based case-control study of patients with community-acquired pneumonia (CAP) noted poor oral health as a risk factor for infection. Similarly, poor oral health has been established as a significant risk factor for aspiration pneumonia among hospitalized patients.[3]
Epidemiology
The variability among patients with aspiration and inconsistent diagnostic practices pose challenges in determining the true incidence of aspiration pneumonia. Data suggests that up to 20% of individuals in the US experience some degree of impaired swallowing, contributing to approximately 0.4% of all hospital admissions being attributed to aspiration pneumonia.[1] The incidence of aspiration pneumonia among patients with CAP ranges between 5% and 15.5% in the US, United Kingdom, and Korea. However, studies from Japan report a notably higher incidence, around 60%, among patients presenting with CAP. Moreover, aspiration pneumonia among patients with hospital-acquired pneumonia (HAP) in Japan is approximately.[2]
In patients under 80, an aspiration event leads to pneumonia only 5% of the time, whereas patients older than 80 develop pneumonia 10% of the time following such an event. Notably, among nursing home residents diagnosed with pneumonia, 18% to 30% of cases are associated with an aspiration event.[3]
It is imperative to recognize that not all aspiration events result in pneumonia. For instance, in cases involving anesthesia, up to 64% of instances did not exhibit any clinical or radiological signs of infection following aspiration events.[4]
Pathophysiology
Aspiration pneumonia occurs when bacteria-rich fluid from the oropharynx or upper GI tract is aspirated into the lungs in sufficient quantities to overcome protective physiologic mechanisms against infection. Intact swallow and cough reflexes prevent aspiration in adequate amounts to produce pneumonia. However, impaired swallow or cough reflexes permit aspirated material to reach the alveoli, causing infection. Even small amounts of aspirated secretions can incite infection in patients with a high bacterial load.[1] Notably, overt aspiration events often go unnoticed, with microaspiration, or silent aspiration, central to the pathophysiological mechanism underlying aspiration pneumonia.[2]
While the bacterial load in microaspirations may be minimal, recurrent microaspirations can progressively lead to recurrent aspiration pneumonia over time due to repetitive epithelial injury caused by frequent aspiration events that denude the pulmonary epithelium. Ultimately, the mucociliary transport mechanism and the macrophage-dependent clearance of aspirated material become compromised. In individuals with compromised immunity, subsequent exposure to aspirated organisms in the alveolar space precipitates infection.[4]
Various interacting mechanisms can contribute to impaired swallowing. In older adults, diminished pharyngeal sensory perception for swallowing and coughing significantly influence impaired swallowing. Additionally, asynchronous breathing patterns during swallowing, characterized by inhalation instead of exhalation, can lead to aspiration. Other predisposing factors include reduced saliva production, ineffective dentition, and delayed laryngeal closure. Furthermore, with aging, the upper esophageal sphincter may decrease in cross-sectional area, resulting in more significant amounts of pharyngeal residue and increasing the risk of aspiration.[1]
In the absence of infection, lung fluid is typically considered sterile. However, recent advancements in culture-independent molecular techniques have identified the lung microbiome, which shares many microbes with the normal flora of the oropharynx. A disequilibrium of oropharyngeal bacterial communities is thought to contribute to decreased lung resistance to colonization and a diminished ability to contain potential pathogens. This notion is supported by the predominance of aerobic gram-negative and gram-positive pathogens as the leading causes of infection in hospitalized patients with aspiration pneumonia.
While anaerobic bacteria were historically considered the dominant cause of aspiration pneumonia, they are now regarded as infrequent causative pathogens.[3] The consensus is that aspiration pneumonia is polymicrobial, with aerobic gram-negative bacilli as the predominant pathogens and aerobic gram-positive organisms as the second most common cause.[1]
History and Physical
Inconsistent definitions and diagnostic practices have posed challenges in delineating a clear clinical profile of patients with aspiration pneumonia. The overlap of aspiration events and the typical clinical presentation further complicates distinguishing aspiration pneumonia from aspiration pneumonitis. Broadly, patients with aspiration pneumonia typically exhibit characteristics such as older age, frailty, malnutrition, and bedridden status, often accompanied by multiple comorbidities, especially cerebrovascular disease.[5]
Patients with aspiration pneumonia commonly exhibit clinical symptoms associated with CAP, including cough, fever, and malaise, which can obscure the distinction between the 2 conditions. In cases where CAP is suspected, obtaining a comprehensive history regarding current or previous dysphagia, instances of aspiration, coughing during eating or drinking, and other medical conditions predisposing to overt or silent aspiration aids in diagnosing aspiration pneumonia.[5]
As outlined earlier, aspiration in patients with pneumonia does not unequivocally signify aspiration pneumonia. However, given the heightened frequency of aspiration events in older adults and the increased pneumonia risk in patients with dysphagia or microaspirations, the current recommendation is to regard aspiration pneumonia as the probable cause of pneumonia in elderly patients, and efforts should be made to evaluate and manage impaired swallowing thoroughly.[5] Conversely, a history of large-volume overt or witnessed aspiration event suggests aspiration pneumonitis rather than aspiration pneumonia.[1]
In most cases, the onset of symptoms in aspiration pneumonia is acute. However, it may present subacutely due to less virulent bacteria. Clinical signs and symptoms commonly observed in patients with aspiration pneumonia include dyspnea, hypoxemia, and fever. One distinguishing feature of aspiration pneumonitis compared to aspiration pneumonia is the occurrence of hyperacute hypoxemia almost immediately in affected patients. This hyperacute hypoxemia can either progress to severe acute lung injury with or without acute respiratory distress syndrome or resolve entirely within 48 hours of onset.[4]
During the initial evaluation, the clinical history should encompass inquiries regarding any episodes of decreased consciousness and swallowing difficulties. Assessing the swallowing efficacy of tablets, solids, and liquids is crucial, especially in older adults. Specific inquiries regarding prior occurrences of pneumonia and periodontal disease should be made. Additionally, obtaining a social history, including details about smoking and alcohol consumption, is imperative for identifying underlying risk factors for aspiration pneumonia.[1]
During the physical examination, cognitive assessment should be conducted in older adults, mainly if there is no history of overt aspiration. Additionally, immediate evaluation for hypoxemia is essential to ensure prompt correction.[1]
Evaluation
The diagnostic workup recommended by the British Thoracic Society for evaluating patients with suspected aspiration pneumonia includes:[1]
- Plain chest radiograph (CXR)
- Computed tomography (CT) of the chest if CXR is inconclusive or if a CT is required to rule out other diagnoses (such as pulmonary embolism)
- Microbiological evaluation of sputum and blood
- Serum electrolytes, albumin, liver enzymes, and complete blood count
- These tests are not diagnostic on their own but are recommended to aid in assessing the severity of the systemic response.
- Additionally, some tests, such as albumin levels, can help evaluate nutritional status and predict prognosis.
A definitive diagnosis of aspiration requires a videofluoroscopy swallowing study (VFSS), also known as a modified barium swallowing study. Aspiration is confirmed if barium is visible beneath the true vocal cords, a phenomenon termed "silent aspiration" if it occurs without throat clearing or coughing. It's important to recognize that aspiration, particularly microaspiration, is an episodic event that cannot be reliably excluded through a single VFSS study.[1]
Other diagnostic studies used to detect aspiration include:[1]
- Fiber optic endoscopic evaluation of swallowing (FEES): This study directly visualizes varying consistencies of food boluses during a swallow.
- Scintigraphy: They are primarily used in research settings, not a useful clinical test at this time.
- Dual-axis accelerometry: Available in specialist centers only
Radiologic Studies
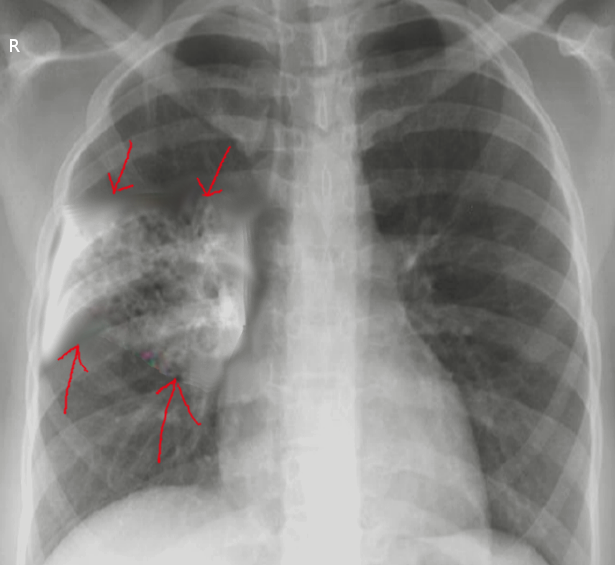
The diagnosis of aspiration pneumonia typically requires radiological evidence of alveolar infiltrates. The presence of infiltrates in dependent areas highly indicates aspiration pneumonia, especially in older adults. However, the specific location of the dependent regions can vary depending on an individual's mobility status. For instance, in mostly upright patients, infiltrates in the basal segments of the lower lobes and the right middle lobe suggest aspiration pneumonia (see Image. Aspiration Pneumonia). Conversely, in predominantly bedridden patients, infiltrates may appear in the superior segments of the lower lobes or posterior parts of the upper lobes (see Image. Ventilator-Associated Aspiration Pneumonia).
In most cases of aspiration pneumonia, the right lung is more frequently affected than the left.[1] However, the presence of a left-sided infiltrate does not exclude aspiration pneumonia. Bilateral lower lobe involvement may be seen in patients who aspirate while upright, while left-sided infiltrates can occur in those who aspirate while in the left lateral decubitus position. Additionally, patients who aspirate while prone may present with right upper lobe infiltrates.[4]
In patients with fluoroscopically documented dysphagia, bronchopneumonia is more commonly observed than lobar pneumonia. Additionally, most (92%) of these patients develop posterior infiltrates. Furthermore, patients with poor performance typically exhibit diffuse rather than focal infiltrates.[6]
Although a plain radiograph of the chest suffices to acquire this information, it may fail to detect an infiltrate in up to 25% of the cases that are subsequently found to have an infiltrate on CT.[1] In a study involving 208 patients with pneumonia, over 60% had aspiration, and the chest radiograph yielded negative results in 28% of them. However, they were subsequently diagnosed with pneumonia upon CT examination.[6]
Ultrasonography also demonstrates high sensitivity and specificity for pneumonia detection. In cases where the CXR is negative, some diagnostic algorithms recommend performing a lung ultrasound before CT to identify the presence of aspiration pneumonia.[3]
Laboratory TestingLaboratory evaluation typically reveals acute inflammation and infection signs, such as an elevated white blood cell count (WBC). However, this may not occur in frail older patients as they may be unable to mount an effective response to infection.[1] Currently, no specific biomarker distinguishes aspiration pneumonia from other diseases or even pneumonitis.
Some experts suggest using serum procalcitonin levels to differentiate aspiration pneumonia from aspiration pneumonitis. Procalcitonin is a biomarker specific for bacterial infections, and an elevation is expected in the case of aspiration pneumonia.[4] However, its performance in critically ill patients to differentiate between aspiration pneumonia and aspiration pneumonitis has been poor.[1]
Alpha-amylase levels in airway secretions have been investigated as potential biochemical markers for aspiration. In a study involving mechanically ventilated patients, elevated alpha-amylase levels were observed in airway secretions; however, their precise relevance to aspiration pneumonia and chemical pneumonitis remains uncertain.[6]
Diagnostic Algorithm
A recent diagnostic algorithm has been proposed to facilitate the diagnosis of aspiration pneumonia and aid in distinguishing it from aspiration pneumonitis. According to the authors of this algorithm, a combination of clinical pneumonia features and characteristic bronchopulmonary findings on radiological assessment is necessary to diagnose aspiration pneumonia.
In frail, older patients presenting with acute respiratory symptoms, with or without fever, along with characteristic radiologic findings, inquiries about the history of known aspiration events should be initiated. Suppose a history of aspiration, along with at least 1 risk factor for oral colonization with a pathogenic or high burden of bacteria, is present. In that case, a definitive diagnosis of aspiration pneumonia can be established.[3]
Risk factors for oral colonization include old age, malnutrition, smoking, dry mouth, poor oral hygiene, and antimicrobial use in the preceding 90 days. Additionally, tracheal cannulation, medications that modify gastric pH (eg, proton pump inhibitors, histamine H2 blockers), enteral nutrition, and inhaled corticosteroids are considered risk factors for oral colonization.[3]
Patients presenting with clinical and radiological findings suggestive of aspiration pneumonia in the absence of a known history of aspiration before a presentation should be diagnosed with aspiration pneumonia if they possess 1 or more risk factors for oral colonization. Risk factors that elevate the likelihood of aspiration include frailty, prior history of stroke, GI disorders, altered mental status, neurologic disorders, or obstructive sleep apnea. Additionally, enteral nutrition, endotracheal intubation, upper GI endoscopy, and recent cardiac arrest are considered risk factors for aspiration.[3]
Patients presenting with clinical and radiologic findings suggestive of aspiration pneumonia without a known history of aspiration before presentation and with at least 1 risk factor for aspiration but without any risk factor for oral colonization should be diagnosed with aspiration pneumonitis.[3]
According to this algorithm, the likelihood of aspiration pneumonia or aspiration pneumonitis is low in cases where typical clinical symptoms and radiologic findings are present without a history of aspiration, aspiration risk factors, or oral colonization risk factors.[3]
Treatment / Management
Clinicians should be aware that the recommendations for managing aspiration pneumonia have evolved. Anaerobic coverage is no longer advised in the empiric treatment of aspiration pneumonia due to the low frequency of actual anaerobic infections causing aspiration pneumonia. Additionally, routine diagnostic techniques often cannot reliably detect anaerobic bacteria, which may lead to inappropriate treatment.
The Infectious Diseases Society of America (IDSA) also notes that routine diagnostic techniques cannot reliably detect anaerobic bacteria. Given the lack of microbial culture guidance, inappropriate treatment will likely occur. Therefore the ISDA suggests anaerobic coverage solely in cases of "classic aspiration pleuropulmonary syndromes" where patients have a history of loss of consciousness (eg, alcohol use, drug overdose, seizures) along with concomitant gingival disease or esophageal motility disorders.
Current guidelines recommend empiric treatment for CAP and maintaining high oxygen tension with mechanical ventilation in situations involving small-volume aspirations, such as during intubation.[7] This approach aims to provide appropriate treatment while minimizing the risk of unnecessary antibiotic use.(B3)
Current guidelines for treating aspiration pneumonia follow recommendations outlined in 2019 by the American Thoracic Society (ATS) and IDSA for managing CAP. The initial step in determining a treatment regimen is to assess the severity of the pneumonia. As per 2019 ATS/IDSA, severe CAP is diagnosed when 1 major criterion or 3 or minor criteria from the following list are present:[8](A1)
- Minor criteria
- Respiratory rate is >30 breaths/min.
- Ratio of PaO2/FIO2 <250
- Multilobar infiltrates are present
- The patient is confused or disoriented
- Serum urea nitrogen level is >20 mg/dL
- WBC <4,000 cells/mL due to the severity of the infection (not due to other causes such as malignancy or chemotherapy)
- Platelet count <100,000/mL
- Core temperature <36 °C
- The patient is hypotensive and requires aggressive fluid resuscitation
- Major criteria
- Patient is in septic shock, requiring vasopressors to maintain adequate mean arterial blood pressure
- Patient is in respiratory failure and requires mechanical ventilation
Ampicillin/sulbactam, carbapenems, or respiratory fluoroquinolones (such as levofloxacin or moxifloxacin) are effective for most patients with community-acquired aspiration pneumonia.[6] According to the 2019 ATS/IDSA guidelines, for adults without severe pneumonia, significant comorbidities, or risk factors for antibiotic-resistant pathogens in the outpatient setting, any 1 of the following regimens is appropriate:[8](A1)
- Amoxicillin 1 g 3 times daily
- Doxycycline 100 mg twice daily
- A macrolide, such as azithromycin 500 mg on the first day, followed by 250 mg daily or clarithromycin 500 mg twice daily, or clarithromycin extended-release 1,000 mg daily)
- Macrolides should only be used in areas where regional pneumococcal resistance to macrolides is <25%.
Patients with comorbidities such as chronic heart disease, chronic liver disease, renal failure, diabetes, chronic lung disease, alcoholism, asplenia, or malignancy with nonsevere pneumonia who are being treated on an outpatient basis the following treatment options are recommended:[8](A1)
- Amoxicillin/clavulanate 500 mg/125 mg 3 times daily, 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily
- A cephalosporin such as cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily, combined with a macrolide (as described previously) or doxycycline
- A cephalosporin and macrolide combination is preferred.
- A respiratory fluoroquinolone such as levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily
Hospitalized patients with nonsevere CAP, without risk factors for methicillin-resistant Staphylococcus aureus (MRSA) infection or Pseudomonas aeruginosa, may be treated with 1 of the following regimens:[8](A1)
- Ampicillin/sulbactam 1.5 to 3 g every 6 hours or cefotaxime 1 to 2 g every 8 hours or ceftriaxone 1 to 2 g daily or ceftaroline 600 mg every 12 hours plus a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily)
- Ampicillin/sulbactam 1.5 to 3 g every 6 hours or cefotaxime 1 to 2 g every 8 hours or ceftriaxone 1 to 2 g daily or ceftaroline 600 mg every 12 hours plus doxycycline 100 mg twice daily
- This option is considered third-line therapy in patients with contraindications to fluoroquinolones and macrolides.
- Levofloxacin 750 mg daily
- Moxifloxacin 400 mg daily
The only difference in the management of inpatient adults with severe CAP without risk factors for MRSA or P aeruginosa, compared to hospitalized patients with nonsevere CAP without these risk factors, is that the former cannot be treated with fluoroquinolone monotherapy or beta-lactam plus doxycycline combination therapy. These regimens have not been studied extensively in this population and are therefore not recommended as empiric therapy for adults with severe CAP.[8](A1)
Patients recently hospitalized (within the last 90 days for at least 5 days), received parenteral antibiotics, and validated risk factors for MRSA or P aeruginosa, should receive empiric coverage for these organisms only if they have severe CAP on presentation. Nonsevere pneumonia in these patients may be treated with the above regimens, with specific testing to rule out these infections.
However, if patients with CAP have previously been infected or colonized with either of these organisms, they should receive empiric coverage regardless of the severity of the pneumonia. MRSA and P aeruginosa coverage may be subsequently discontinued if culture or nasal testing does not isolate these organisms.[8] In patients with validated risk factors for MRSA or P aeruginosa or a prior history of infections secondary to these organisms, treatment with piperacillin/tazobactam, cefepime, imipenem, or meropenem is required, in combination with vancomycin or linezolid.[6] (A1)
A minimum of 5 days of therapy is required for the treatment.[6][8] Longer durations may be considered for patients with slow clinical response, necrotizing pneumonia, lung abscess, or empyema.[6](A1)
Dedicated anaerobic coverage is not advised in patients with suspected aspiration pneumonia. While patients with suspected aspiration pneumonia and accompanying lung abscesses or empyema may be considered for anaerobic coverage, it is essential to note that there is very low-quality evidence supporting this recommendation.[8] (A1)
Of particular concern is the use of clindamycin to cover for anaerobes, as it increases the risk for Clostridium difficile colitis. Clinical data shows no significant difference between ampicillin/sulbactam, clindamycin, and carbapenems for treating suspected aspiration pneumonia. Additionally, moxifloxacin has demonstrated similar efficacy in treating aspiration pneumonia compared to ampicillin/sulbactam, with a clinical response rate of 66.7%.[6]
Clindamycin is recommended only as additional therapy to provide better anaerobic coverage in patients with a high risk of predominantly anaerobic infection, such as those with severe periodontal disease coupled with necrotizing pneumonia or lung abscesses.[6] However, its use should be carefully considered due to the associated risk of C difficile colitis.
Therapy with glucocorticoids has been extensively studied in patients with aspiration syndromes, but their use has not shown consistent clinical benefit in patients with aspiration-associated pleuropulmonary disease. According to current recommendations, glucocorticoid therapy does not manage aspiration pneumonia or aspiration pneumonitis.[6][8](A1)
The British Thoracic Society offers similar recommendations for managing aspiration pneumonia, emphasizing the polymicrobial nature of aspiration pneumonia and the necessity for broader-spectrum antibiotics for effective treatment. However, citing ecological concerns associated with the use of cephalosporins, the British Thoracic Society guidelines suggest initiating therapy with amoxicillin for most patients with aspiration pneumonia. In cases of penicillin allergy, alternatives such as respiratory fluoroquinolone, a macrolide, or a tetracycline may be considered.[1]
Adequate oxygenation is another essential component of treatment in patients with treatment in patients with aspiration pneumonia. The British Thoracic Society guidelines recommend maintaining a 94% to 98% target oxygen saturation for most patients. In acutely ill patients with a higher disease severity, oxygen saturation of 94% to 96% may be optimal. However, exceptions should be made for patients at risk for hypercapnia due to underlying comorbidities, with a target oxygenation range between 88% and 92% as clinically appropriate.[1]
Differential Diagnosis
In the differential diagnosis of aspiration pneumonia, several conditions warrant consideration, and it is crucial to differentiate aspiration pneumonia from these entities:[4]
- Aspiration pneumonitis
- CAP
- Acute respiratory distress syndrome
- Viral Pneumonia
- Negative-pressure pulmonary edema: Presents with bilateral symmetric lung infiltrates and occurs due to breathing against a closed airway. It can happen during events such as general anesthesia, choking, or near drowning.[6]
Prognosis
Patients with CAP who have an increased aspiration risk are noted to have higher in-hospital and 30-day mortality. Aspiration risk also increases recurrent pneumonia risk and all-cause readmission rates. Aspiration pneumonia is identified as an independent risk factor for these outcomes, although the lack of robust data prevents specific predictions relating to aspiration pneumonia.[9]
Older adults aged over 80 with pneumonia and those with aspiration pneumonia exhibited higher mortality rates, elevated sodium levels, and poorer renal function compared to age-matched controls with pneumonia but without evidence of aspiration.[6]
In the US, aspiration pneumonia caused an average of 58,576 deaths per year between 1999 and 2017, with individuals aged 75 or older accounting for 76% of deaths attributable to aspiration pneumonia.[10] Among hospitalized patients with aspiration pneumonia, the mortality rate is approximately 10% to 15%, with the highest risk of poor outcomes observed in patients with older age. Notably, patients with head and neck cancer have a mortality rate of around 20% due to aspiration pneumonia.[1]
In patients with Parkinson disease, aspiration pneumonia is associated with a high risk of mortality, even in the early stages of the disease. A large nationwide study from South Korea revealed that two-thirds of patients with Parkinson disease died within 1 year of the first occurrence of AP.[11]
As expected, the severity of aspiration pneumonia is a significant predictor of mortality. However, another predictor of mortality in patients with aspiration pneumonia is the underlying nutritional status. Early assessment of nutritional status is recommended in patients with aspiration pneumonia to predict prognosis and facilitate improved clinical outcomes.[12]
Complications
The most prevalent complications of aspiration pneumonia include lung abscesses and empyema. Clinical reports have been published documenting lung abscesses and empyema in patients with aspiration pneumonia attributed to anaerobes and gram-negative bacteria.[13][14] Prompt antibiotic initiation with appropriate regimens tailored to the patient's risk of drug-resistant infections is recommended to decrease the risk of these complications.
Moreover, patients with aspiration pneumonia face risks of malnutrition and dehydration, often linked to the underlying condition predisposing them to aspiration. However, there is an additional risk for malnutrition and dehydration in patients with aspiration pneumonia placed on modified diets, especially if thickened liquids are utilized. Patients may restrict their oral intake to avoid thickened liquids, leading to significantly reduced intake levels (as low as 22% of the daily recommended amount in some cases).[15] Diligent monitoring and community follow-up are imperative to ensure patients maintain sufficient levels of hydration and nutrition on modified diets to prevent these complications.
Recurrent hospitalizations and reduced quality of life, particularly among older frail individuals, are common occurrences with aspiration pneumonia. Patients with dysphagia have a higher hospital readmission rate for pneumonia, reaching 6.7 per 100 person-years compared to 3.67 per 100 person-years for those without dysphagia or aspiration history.[15] Multilevel clinical support from nutritionists, speech-language pathologists, physical therapists, and nurses is indispensable to enhance the quality of life for these individuals and decrease their risk of hospital readmissions.
Deterrence and Patient Education
Regular dysphagia screening and testing play a crucial role in identifying and managing swallowing difficulties, thereby mitigating the risk of aspiration pneumonia and improving overall patient outcomes. The European Stroke Organisation and European Society for Swallowing Disorders guidelines advocate for formal dysphagia screening in all patients with acute stroke. This aids in the early diagnosis of dysphagia, prevents post-stroke aspiration pneumonia, and reduces the risk of early mortality.[16]
Similarly, in patients with Parkinson disease, early screening and ongoing assessment for dysphagia are essential components of comprehensive care, aiding in timely intervention and reducing the risk of aspiration-related complications.[17] These proactive measures also help tailor interventions to address swallowing impairments and reduce the likelihood of aspiration-related complications.
Preventive strategies for aspiration pneumonia should adopt a comprehensive approach involving collaboration among various healthcare professionals. Speech-language pathologists and dieticians play a crucial role in restoring effective swallowing and cough mechanisms, while nursing staff and oral hygienists focus on reducing the oral bacterial load. Additionally, nutritionists are instrumental in ensuring adequate hydration and caloric intake, especially when patients are prescribed modified consistencies of solids and liquids.[1]
Patients at risk for aspiration syndromes should undergo evaluation before experiencing an overt aspiration event or aspiration pneumonia. Clinical assessment by nurses and triage staff is paramount in identifying patients with a high aspiration risk. Many different screening tools are available, with no clear evidence identifying the superiority of one screening tool over another. In frail older patients who do not have identifiable risks of aspiration, such as an acute stroke, simple screening tools should be implemented during initial medical contact to detect aspiration risk. One recent study identified the following 4 questions to screen for aspiration risk:[18]
- Do you cough and choke when you eat and drink?
- Does it take longer to eat your meals than it used to?
- Have you changed the type of food that you eat?
- Does your voice change after eating or drinking?
An affirmative answer to any of the above questions implies impaired swallowing. The pilot study for this screen reported very high sensitivity with a specificity of 80.4%. The advantage of this screening tool is that it is easy to use and consists of very few items that may be administered by any individual in the healthcare setting without any specific training.[18]
When aspiration is identified, multiple strategies are utilized to prevent the risk of aspiration pneumonia. The chin-tuck or chin-down method provides physical support to the pharyngolaryngeal musculature by asking the patient to touch the chin against the chest during swallowing. Specific populations, especially those of Asian descent, also benefit from using angiotensin-converting enzyme (ACE) inhibitors. ACE inhibitors prevent the breakdown of substance P and help preserve cough mechanisms, thus decreasing the risk of AP in these patients.[1]
Other strategies, such as expiratory muscle training in patients with Parkinson disease or voice exercises in patients with glottal closure, have also shown benefits in reducing aspiration frequency. However, the studies supporting their use are small.[1]
In several studies, oral care provided via mechanical techniques (toothbrush as opposed to chlorhexidine rinses) has shown reduced aspiration pneumonia frequency and deaths. The British Thoracic Society guidelines recommend nonfoaming fluoride toothpaste in these patients to minimize the risk of aspiration.[1]
Modifying the viscosity of fluids and the texture of food in patients with impaired swallowing is used to decrease the risk of aspiration pneumonia worldwide, but little clinical data supports this practice. The current literature does not identify any convincing evidence to suggest that texture-modified food and thickened liquids prevent aspiration pneumonia when used as a stand-alone therapy. However, when modified diets are combined with compensatory postural techniques (such as the chin-tuck maneuver) and therapeutic exercises to strengthen pharyngolaryngeal musculature, they have demonstrated significant clinical impact in reducing the incidence of aspiration pneumonia.[19]
It is important to note that modified diets can lead to detrimental side effects. They have been shown to increase the risk of malnutrition, dehydration, and urinary tract infections, especially in elderly patients with underlying neurological disorders.[19] In addition, thickened textures can increase pharyngeal residue. Caution is therefore advised when implementing modified diets in the management of oropharyngeal dysphagia. Careful monitoring to minimize the risk of dehydration and malnutrition is warranted in all individuals on a modified diet. Smaller volumes are recommended in these patients to reduce residue and subsequent aspiration events.[1]
Balancing adequate nutrition while minimizing the risk of aspiration is of prime importance. The British Thoracic Society guidelines suggest enteral feeding in patients with no oral intake for more than 3 days or if less than 50% of nutritional requirement is met for more than 10 days.[1]
Pearls and Other Issues
Key points to keep in mind about aspiration pneumonia include the following:
- Aspiration pneumonia occurs when oral or gastric contents are aspirated into the lungs, leading to infection. It often results from impaired swallowing or protective airway reflexes.
- The common risk factors associated with aspiration pneumonia include advanced age, dysphagia, altered mental status, neurological disorders, and conditions that affect the gag reflex or esophageal motility.
- Symptoms may be similar to those of CAP but with a higher risk of recurrent episodes.
- CXR findings typically include infiltrates in the dependent lung segments.
- Potential complications of aspiration pneumonia are lung abscesses, empyema, and respiratory failure.
- Preventive measures to reduce the risk of aspiration pneumonia include proper positioning during feeding, swallowing assessments, and addressing underlying conditions contributing to dysphagia.
- Certain patient populations, such as the older individuals and those with neurological conditions like Parkinson disease or stroke, are at higher risk for aspiration pneumonia and may require tailored management approaches.
Enhancing Healthcare Team Outcomes
Aspiration pneumonia presents a significant health challenge, particularly for vulnerable populations such as older individuals and those with compromised mobility. Contrary to historical beliefs, aspiration pneumonia is not solely caused by overt aspiration events leading to anaerobic infections. Instead, most of the population experiences silent microaspirations, especially during sleep. These microaspirations and certain risk factors contribute to recurrent polymicrobial aspiration pneumonia, which is rarely anaerobic. Recognizing these nuances is crucial for accurately identifying aspiration pneumonia and implementing effective management strategies for affected patients.
To effectively mitigate the adverse outcomes associated with aspiration pneumonia, it is crucial to proactively identify individuals at risk before the onset of the condition. This necessitates the collaboration of a diverse team of healthcare professionals, including clinicians, such as physicians and advanced practitioners, nurses, speech-language pathologists, nutritionists, and pharmacists.
Clinical nurses play a pivotal role in screening for impaired swallowing by using highly sensitive assessment tools to detect any signs of compromised swallowing function. Subsequently, speech-language pathologists can conduct thorough evaluations to diagnose swallowing impairments and provide tailored recommendations to minimize the risk of aspiration. Clinical nurses must ensure patients comprehend and can execute these recommendations effectively.
Nutritionists contribute by ensuring that patients receive sufficient hydration and caloric intake, especially when adhering to modified diets. Pharmacists play a vital role in mitigating adverse medication effects, such as sedation, which could elevate the risk of aspiration. Moreover, they assist in ensuring patients receive appropriate medication formulations, particularly when pills need to be altered.
Through collaborative efforts across these disciplines, the interprofessional team can significantly reduce the incidence of aspiration pneumonia and enhance overall patient outcomes.
Media
(Click Image to Enlarge)
Ventilator-Associated Aspiration Pneumonia. Chest radiograph showing ventilator-associated aspiration pneumonia.
Melvil, Public Domain, via Wikimedia Commons.
(Click Image to Enlarge)
References
Simpson AJ, Allen JL, Chatwin M, Crawford H, Elverson J, Ewan V, Forton J, McMullan R, Plevris J, Renton K, Tedd H, Thomas R, Legg J BTS clinical statement on aspiration pneumonia. Thorax. 2023 Feb [PubMed PMID: 36863772]
Teramoto S The current definition, epidemiology, animal models and a novel therapeutic strategy for aspiration pneumonia. Respiratory investigation. 2022 Jan [PubMed PMID: 34782300]
Level 3 (low-level) evidenceAlmirall J, Boixeda R, de la Torre MC, Torres A Aspiration pneumonia: A renewed perspective and practical approach. Respiratory medicine. 2021 Aug-Sep [PubMed PMID: 34087609]
Level 3 (low-level) evidenceKošutova P, Mikolka P. Aspiration syndromes and associated lung injury: incidence, pathophysiology and management. Physiological research. 2021 Dec 30:70(Suppl4):S567-S583 [PubMed PMID: 35199544]
Yoshimatsu Y, Melgaard D, Westergren A, Skrubbeltrang C, Smithard DG. The diagnosis of aspiration pneumonia in older persons: a systematic review. European geriatric medicine. 2022 Oct:13(5):1071-1080. doi: 10.1007/s41999-022-00689-3. Epub 2022 Aug 25 [PubMed PMID: 36008745]
Level 1 (high-level) evidenceMandell LA, Niederman MS. Aspiration Pneumonia. The New England journal of medicine. 2019 Feb 14:380(7):651-663. doi: 10.1056/NEJMra1714562. Epub [PubMed PMID: 30763196]
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG, Infectious Diseases Society of America, American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007 Mar 1:44 Suppl 2(Suppl 2):S27-72 [PubMed PMID: 17278083]
Level 3 (low-level) evidenceMetlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, Griffin MR, Metersky ML, Musher DM, Restrepo MI, Whitney CG. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. American journal of respiratory and critical care medicine. 2019 Oct 1:200(7):e45-e67. doi: 10.1164/rccm.201908-1581ST. Epub [PubMed PMID: 31573350]
Level 1 (high-level) evidenceKomiya K, Rubin BK, Kadota JI, Mukae H, Akaba T, Moro H, Aoki N, Tsukada H, Noguchi S, Shime N, Takahashi O, Kohno S. Prognostic implications of aspiration pneumonia in patients with community acquired pneumonia: A systematic review with meta-analysis. Scientific reports. 2016 Dec 7:6():38097. doi: 10.1038/srep38097. Epub 2016 Dec 7 [PubMed PMID: 27924871]
Level 1 (high-level) evidenceGupte T, Knack A, Cramer JD. Mortality from Aspiration Pneumonia: Incidence, Trends, and Risk Factors. Dysphagia. 2022 Dec:37(6):1493-1500. doi: 10.1007/s00455-022-10412-w. Epub 2022 Jan 31 [PubMed PMID: 35099619]
Won JH, Byun SJ, Oh BM, Park SJ, Seo HG. Risk and mortality of aspiration pneumonia in Parkinson's disease: a nationwide database study. Scientific reports. 2021 Mar 23:11(1):6597. doi: 10.1038/s41598-021-86011-w. Epub 2021 Mar 23 [PubMed PMID: 33758213]
Yanagita Y, Arizono S, Tawara Y, Oomagari M, Machiguchi H, Yokomura K, Katagiri N, Iida Y. The severity of nutrition and pneumonia predicts survival in patients with aspiration pneumonia: A retrospective observational study. The clinical respiratory journal. 2022 Jul:16(7):522-532. doi: 10.1111/crj.13521. Epub 2022 Jul 5 [PubMed PMID: 35789107]
Level 2 (mid-level) evidenceKumar NR, Norwood BS. Sphingomonas Paucimobilis Pneumonia Complicated by Empyema in an Immunocompetent Patient: A Case Report and Concise Review of Literature. Cureus. 2022 May:14(5):e24820. doi: 10.7759/cureus.24820. Epub 2022 May 8 [PubMed PMID: 35693369]
Level 3 (low-level) evidenceMartinez K, Mangat GK, Sherwani N, Glover DO M, Silver Md M. Veillonella Intrapulmonary Abscess With Empyema. Cureus. 2023 Sep:15(9):e45210. doi: 10.7759/cureus.45210. Epub 2023 Sep 14 [PubMed PMID: 37842426]
Baijens LW, Clavé P, Cras P, Ekberg O, Forster A, Kolb GF, Leners JC, Masiero S, Mateos-Nozal J, Ortega O, Smithard DG, Speyer R, Walshe M. European Society for Swallowing Disorders - European Union Geriatric Medicine Society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clinical interventions in aging. 2016:11():1403-1428 [PubMed PMID: 27785002]
Dziewas R, Michou E, Trapl-Grundschober M, Lal A, Arsava EM, Bath PM, Clavé P, Glahn J, Hamdy S, Pownall S, Schindler A, Walshe M, Wirth R, Wright D, Verin E. European Stroke Organisation and European Society for Swallowing Disorders guideline for the diagnosis and treatment of post-stroke dysphagia. European stroke journal. 2021 Sep:6(3):LXXXIX-CXV. doi: 10.1177/23969873211039721. Epub 2021 Oct 13 [PubMed PMID: 34746431]
Cosentino G, Avenali M, Schindler A, Pizzorni N, Montomoli C, Abbruzzese G, Antonini A, Barbiera F, Benazzo M, Benarroch EE, Bertino G, Cereda E, Clavè P, Cortelli P, Eleopra R, Ferrari C, Hamdy S, Huckabee ML, Lopiano L, Marchese Ragona R, Masiero S, Michou E, Occhini A, Pacchetti C, Pfeiffer RF, Restivo DA, Rondanelli M, Ruoppolo G, Sandrini G, Schapira AHV, Stocchi F, Tolosa E, Valentino F, Zamboni M, Zangaglia R, Zappia M, Tassorelli C, Alfonsi E. A multinational consensus on dysphagia in Parkinson's disease: screening, diagnosis and prognostic value. Journal of neurology. 2022 Mar:269(3):1335-1352. doi: 10.1007/s00415-021-10739-8. Epub 2021 Aug 21 [PubMed PMID: 34417870]
Level 3 (low-level) evidenceTsang K, Lau ES, Shazra M, Eyres R, Hansjee D, Smithard DG A New Simple Screening Tool-4QT: Can It Identify Those with Swallowing Problems? A Pilot Study. Geriatrics (Basel, Switzerland). 2020 Feb 27 [PubMed PMID: 32120993]
Level 3 (low-level) evidenceO'Keeffe ST. Use of modified diets to prevent aspiration in oropharyngeal dysphagia: is current practice justified? BMC geriatrics. 2018 Jul 20:18(1):167. doi: 10.1186/s12877-018-0839-7. Epub 2018 Jul 20 [PubMed PMID: 30029632]